| General Information of Drug (ID:
DR1204) |
| Drug Name |
RO-7-0207
|
| Synonyms |
Madelen; Ornidazol; Ornidazole; Ornidazol [INN-Spanish]; Ornidazole Levo-; Ornidazole [USAN:INN]; Ornidazolum; Ornidazolum [INN-Latin]; Ro 7-0207; Ro-70207; Ro7-0207; Tiberal; 1-(2-Hydroxy-3-chloropropyl)-2-methyl-5-nitroimidazole; 1-(3-Chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole; 1-Chloro-3-(2-methyl-5-nitro-1H-imidazol-1-yl)-2-propanol; 1-chloro-3-(2-methyl-5-nitro-1H-imidazol-1-yl)propan-2-ol; 16773-42-5; BRN 0614299; C7H10ClN3O3; CCRIS 9030; EINECS 240-826-0; MLS000028628; NSC 95075
|
| Indication |
Colon cancer
[ICD11: 2B90]
|
Phase 3
|
[1]
|
| Structure |
|
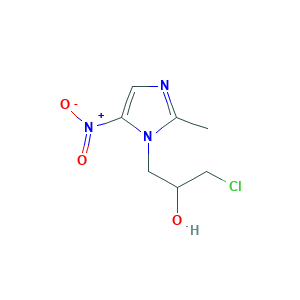 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
219.62 |
Topological Polar Surface Area |
83.9 |
| Heavy Atom Count |
14 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 28061
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D0BE
- Formula
- C7H10ClN3O3
- Canonical SMILES
- CC1=NC=C(N1CC(CCl)O)[N+](=O)[O-]
- InChI
- 1S/C7H10ClN3O3/c1-5-9-3-7(11(13)14)10(5)4-6(12)2-8/h3,6,12H,2,4H2,1H3
- InChIKey
- IPWKIXLWTCNBKN-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


