| General Information of Drug (ID:
DR1343) |
| Drug Name |
Procainamide hydrochloride
|
| Synonyms |
Procainamida; Procainamida [INN-Spanish]; Procainamide [INN:BAN]; Procainamidum; Procainamidum [INN-Latin]; Procainamide (hydrochloride); Procainamide Hcl; Procaine amide hydrochloride; Procainhydrochlorid; Procaini hydrochloridum; Procainii chloridum; Procamide hydrochloride; Procan SR; Procan-SR hydrochloride; Procapan hydrochloride; Procardyl hydrochloride; Promide hydrochloride; Supicane amide hydrochloride; procainamide hydrochloride; 4-amino-N-[2-(diethylamino)ethyl]benzamide hydrochloride; 614-39-1; CCRIS 7143; UNII-SI4064O0LX; Novocamid hydrochloride; Procaine amide; Procamide; Procapan (free base); 4-Amino-N-(2-(diethylamino)ethyl)benzamide; 4-Amino-N-[2-(diethylamino)ethyl]benzamide; 4-amino-N-(2-diethylaminoethyl)benzamide; UNII-L39WTC366D; p-Amino-N-(2-diethylaminoethyl)benzamide; p-Aminobenzoic diethylaminoethylamide; Biocoryl; Novocainamid; Novocainamide; Novocaine amide; Novocamid; PROCAINAMIDE
|
| Indication |
Ventricular tachyarrhythmia
[ICD11: BC71]
|
Approved
|
[1]
|
| Structure |
|
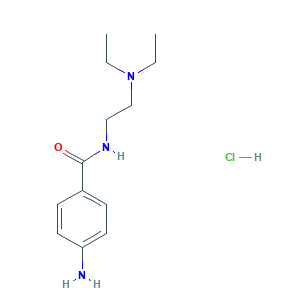 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
271.78 |
Topological Polar Surface Area |
58.4 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 66068
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U5SI
- Formula
- C13H22ClN3O
- Canonical SMILES
- CCN(CC)CCNC(=O)C1=CC=C(C=C1)N.Cl
- InChI
- 1S/C13H21N3O.ClH/c1-3-16(4-2)10-9-15-13(17)11-5-7-12(14)8-6-11;/h5-8H,3-4,9-10,14H2,1-2H3,(H,15,17);1H
- InChIKey
- ABTXGJFUQRCPNH-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


