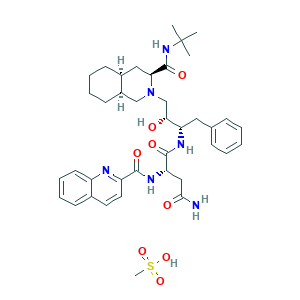
| Synonyms |
Saquinavir mesilate; Saquinavir mesylate; Saquinavir mesylate (AIDS Initiative); Saquinavir monomethanesulfonate salt; Saquinavir, Mesylate; UHB9Z3841A; UNII-UHB9Z3841A; (S)-N-((alphaS)-alpha-((1R)-2-((3S,4aS,8aS)-3-(tert-Butylcarbamoyl)octahydro-2(1H)-isoquinolyl)-1-hydroxyethyl)phenethyl)-2-quinaldamidosuccinamide monomethanesulfonate (salt); 149845-06-7; CHEBI:32121; DSSTox_CID_3835; DSSTox_GSID_23835; DSSTox_RID_77202; NCGC00091469-01; Ro 31-8959/003; (2s)-N-[(2s,3r)-4-[(2s,3s,4as,8as)-3-(Tert-Butylcarbamoyl)-3,4,4a,5,6,7,8,8a-Octahydro-1h-Isoquinolin-2-Yl]-3-Hydroxy-1-Phenyl-Butan-2-Yl]-2-(Quinolin-2-Ylcarbonylamino)butanediamide; 127779-20-8; CHEBI:63621; Fortovase (TN); Fortovase(TM); HSDB 7161; L3JE09KZ2F; ROC; Ro 318959; Ro-31-8959; Ro-318959000; SAQUINAVIR; UNII-L3JE09KZ2F; saguinavir
|
| Cross-matching ID |
- PubChem CID
- 60934
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0WI3T
- Formula
- C39H54N6O8S
- Canonical SMILES
- CC(C)(C)NC(=O)C1CC2CCCCC2CN1CC(C(CC3=CC=CC=C3)NC(=O)C(CC(=O)N)NC(=O)C4=NC5=CC=CC=C5C=C4)O.CS(=O)(=O)O
- InChI
- 1S/C38H50N6O5.CH4O3S/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29;1-5(2,3)4/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49);1H3,(H,2,3,4)/t26-,27+,30-,31-,32-,33+;/m0./s1
- InChIKey
- IRHXGOXEBNJUSN-YOXDLBRISA-N
|