Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1517) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Sumatriptan succinate
|
|||||
| Synonyms |
Sumadol; Sumatrin; Sumatriptan (succinate); Sumatriptan succinate [USAN]; Sumavel DosePro; Sumigrene; Suminat; Sumitrex; UNII-J8BDZ68989; mitrex; sumatriptan hydrogen succinate; 1-(3-(2-(Dimethylamino)ethyl)-1H-indol-5-yl)-N-methylmethanesulfonamide succinate; 103628-48-4; Alsuma; Antibet; Arcoiran; CHEBI:64359; Diletan; GR 43175; GR 43175C; GR-43175C; Imigran; Imijekt; Imitrex; J8BDZ68989; MFCD00902856; Micralgin; Migmax; Migratan; Migratriptan; Novelian; Permicran; SUMATRIPTAN SUCCINATE
|
|||||
| Indication | Migraine [ICD11: 8A80] | Approved | [1] | |||
| Structure |
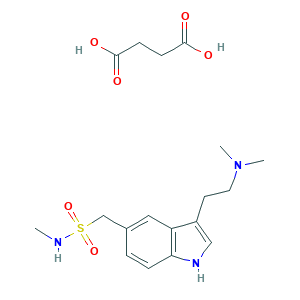 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 413.5 | Topological Polar Surface Area | 148 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 8 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

