| General Information of Drug (ID:
DR1669) |
| Drug Name |
Vandetanib
|
| Synonyms |
Vandetanib; Caprelsa; GNF-PF-2188; YO460OQ37K; ZD 6474; ZD-6474; ZD6474; Zactima; vandetanib (zd6474); 443913-73-3; C22H24BrFN4O2; CHEBI:49960; CHEMBL24828; N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-((1-methyl-4-piperidinyl)methoxy)-4-quinazolinamine; N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-((1-methylpiperidin-4-yl)methoxy)quinazolin-4-amine; N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)methoxy]quinazolin-4-amine; NCGC00167513-01; UNII-YO460OQ37K
|
| Indication |
Thyroid cancer
[ICD11: 2D10]
|
Approved
|
[1]
|
| Structure |
|
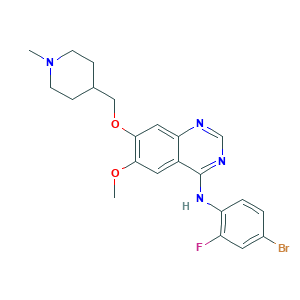 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
475.4 |
Topological Polar Surface Area |
59.5 |
| Heavy Atom Count |
30 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 3081361
- PubChem SID
-
8030012
; 8034317
; 9471114
; 12015522
; 14716916
; 14761077
; 36412170
; 46394288
; 47208064
; 49742597
; 50068212
; 50100123
; 50112766
; 50599279
; 53789193
; 57354678
; 85246147
; 91147360
; 92721418
; 93309619
; 93581029
; 93692935
; 99436956
; 99445235
; 103194628
; 103905338
; 111682639
; 123110212
; 124756961
; 124772087
; 124893337
; 124893338
; 125163767
; 125350704
; 126619162
; 126651857
; 126661732
; 126731545
; 127330006
; 127330007
; 131333378
; 131408693
; 134339002
; 134964431
; 135228305
; 135685324
; 135685325
; 135685344
; 135697656
; 135727395
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G6QF
- Formula
- C22H24BrFN4O2
- Canonical SMILES
- CN1CCC(CC1)COC2=C(C=C3C(=C2)N=CN=C3NC4=C(C=C(C=C4)Br)F)OC
- InChI
- 1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27)
- InChIKey
- UHTHHESEBZOYNR-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


