| General Information of Drug (ID:
DR1677) |
| Drug Name |
Vemurafenib
|
| Synonyms |
Vemurafenib; Vemurafenib (PLX4032); Vemurafenib (PLX4032, RG7204); Zelboraf; PLX 4032; PLX-4032; PLX4032; RG 7204; RG-7204; RG7204; RO 5185426; RO5185426; 1029872-54-5; 207SMY3FQT; 918504-65-1; CHEBI:63637; N-(3-(5-(4-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl)propane-1-sulfonamide; N-(3-{[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl}-2,4-difluorophenyl)propane-1-sulfonamide; NSC761431; UNII-207SMY3FQT
|
| Indication |
Melanoma
[ICD11: 2C30]
|
Approved
|
[1]
|
| Structure |
|
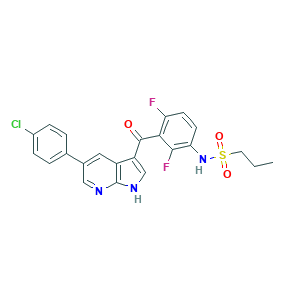 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
489.9 |
Topological Polar Surface Area |
100 |
| Heavy Atom Count |
33 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 42611257
- PubChem SID
-
86450036
; 99207986
; 99344329
; 99436933
; 104245715
; 123055406
; 123393726
; 124757131
; 125163935
; 125240992
; 125312523
; 125477821
; 126731467
; 131408691
; 131480743
; 134213431
; 135267495
; 135360056
; 135611115
; 135626717
; 135686206
; 135686207
; 135686222
; 135686223
; 135727433
; 136367377
; 136920367
; 137241152
; 137275900
; 138196197
; 152258377
; 160647214
; 160837190
; 162011358
; 162037528
; 162201724
; 163098027
; 163345507
; 163390276
; 164041819
; 164193918
; 164766097
; 164834160
; 165826640
; 170483525
; 170498105
; 172087033
; 172914376
; 174531480
; 175267423
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y9EW
- Formula
- C23H18ClF2N3O3S
- Canonical SMILES
- CCCS(=O)(=O)NC1=C(C(=C(C=C1)F)C(=O)C2=CNC3=C2C=C(C=N3)C4=CC=C(C=C4)Cl)F
- InChI
- 1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28)
- InChIKey
- GPXBXXGIAQBQNI-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


