| General Information of Drug (ID:
DR1771) |
| Drug Name |
Adefovir dipivoxil
|
| Prodrug Info |
Adefovir dipivoxil is the prodrug of Adefovir
|
| Synonyms |
Adefovir dipivoxil (USAN); Adefovir dipivoxil [USAN]; Adefovir dipivoxyl; Adefovir pivoxil; Adefovirdipivoxl; Hepsera; Preveon; U6Q8Z01514; bis-POM PMEA; (((2-(6-Amino-9H-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) 2,2-dimethylpropanoate; 142340-99-6; ADEFOVIR DIPIVOXIL; Adefovir Dipivoxil (Preveon, Hepsera); C20H32N5O8P; GS 0840; GS 840; GS-0840; GS-840; MFCD00869897; NCGC00164624-01; UNII-U6Q8Z01514; [2-(6-aminopurin-9-yl)ethoxymethyl-(2,2-dimethylpropanoyloxymethoxy)phosphoryl]oxymethyl 2,2-dimethylpropanoate
|
| Indication |
Viral hepatitis
[ICD11: 1E51]
|
Approved
|
[1]
|
| Structure |
|
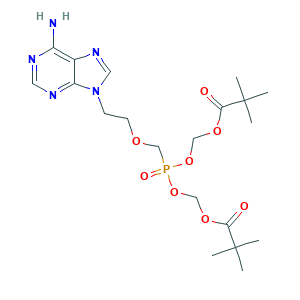 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
501.5 |
Topological Polar Surface Area |
167 |
| Heavy Atom Count |
34 |
Rotatable Bond Count |
15 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
12 |
| Cross-matching ID |
- PubChem CID
- 60871
- PubChem SID
-
612309
; 7848718
; 8187107
; 11528768
; 12014790
; 14908863
; 26758046
; 43118206
; 46507520
; 49830940
; 57314164
; 71824987
; 74485826
; 87244050
; 87245424
; 92712536
; 103240599
; 104253333
; 104321855
; 109692937
; 118048871
; 119525323
; 124757452
; 125164256
; 125340236
; 126592990
; 126630083
; 126656634
; 126665844
; 129613204
; 131298815
; 134222429
; 134337388
; 134338819
; 134340371
; 135018282
; 135565514
; 135692243
; 135727110
; 136345656
; 136368131
; 136946538
; 136949103
; 137006009
; 142181502
; 143493341
; 144115540
; 144180310
; 144205796
; 152242352
- CAS Number
-
- TTD Drug ID
- D0ML1F
- Formula
- C20H32N5O8P
- Canonical SMILES
- CC(C)(C)C(=O)OCOP(=O)(COCCN1C=NC2=C(N=CN=C21)N)OCOC(=O)C(C)(C)C
- InChI
- 1S/C20H32N5O8P/c1-19(2,3)17(26)30-11-32-34(28,33-12-31-18(27)20(4,5)6)13-29-8-7-25-10-24-14-15(21)22-9-23-16(14)25/h9-10H,7-8,11-13H2,1-6H3,(H2,21,22,23)
- InChIKey
- WOZSCQDILHKSGG-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


