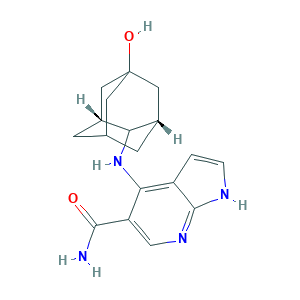
| Synonyms |
Peficitinib; Peficitinib (USAN/INN); Peficitinib [USAN:INN]; SB16834; SC-17960; HPH1166CKX; SCHEMBL1154418; SCHEMBL1154421; SCHEMBL4447032; SCHEMBL9990240; SCHEMBL9990248; 4-[[(1R,3S)-5-hydroxy-2-adamantyl]amino]-1H-pyrrolo[2,3-b]pyridine-5-carboxamide; 4-[[(1S,3R)-5-oxidanyl-2-adamantyl]amino]-1H-pyrrolo[2,3-b]pyridine-5-carboxamide; 944118-01-8; 9T6; ASP 015K; ASP-015K; ASP015K; BCP18465; BDBM50124208; CHEMBL3137308; CS-5393; DB11708; GTPL8315; HY-19568; Peficitinb (ASP015K, JNJ-54781532); SCHEMBL17645135; UNII-HPH1166CKX
|
| Cross-matching ID |
- PubChem CID
- 57928403
- PubChem SID
-
137951042
; 137951123
; 164180739
; 173334224
; 194147758
; 227392433
; 227392436
; 230374902
; 235335272
; 235335280
; 244451093
; 245539868
; 246562931
; 252166527
- CAS Number
-
- TTD Drug ID
- D06EIC
- Formula
- C18H22N4O2
- Canonical SMILES
- C1C2CC3CC(C2)(CC1C3NC4=C5C=CNC5=NC=C4C(=O)N)O
- InChI
- 1S/C18H22N4O2/c19-16(23)13-8-21-17-12(1-2-20-17)15(13)22-14-10-3-9-4-11(14)7-18(24,5-9)6-10/h1-2,8-11,14,24H,3-7H2,(H2,19,23)(H2,20,21,22)/t9?,10-,11+,14?,18?
- InChIKey
- DREIJXJRTLTGJC-JQCLMNFQSA-N
|