| Synonyms |
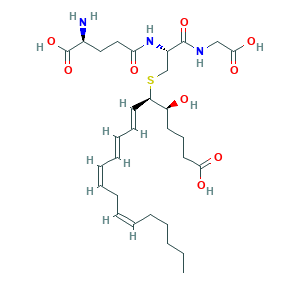
LTC (sub 4); Leukotriene C; Leukotriene C(sub 1); Leukotriene C(sub 4); leukotriene C4; (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-[[(4S)-4-amino-5-hydroxy-5-oxopentanoyl]amino]-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-5-hydroxyicosa-7,9,11,14-tetraenoic acid; 2CU6TT9V48; 5S,6R-Ltc(sub 4); 5S-hydroxy,6R-(S-glutathionyl),7E,9E,11Z,14Z-eicosatetraenoic acid; 72025-60-6; C30H47N3O9S; CHEBI:16978; GWNVDXQDILPJIG-NXOLIXFESA-N; LTC4; LTX; UNII-2CU6TT9V48
|
| Cross-matching ID |
- PubChem CID
- 5280493
- PubChem SID
-
5241
; 4266071
; 7978470
; 8144736
; 8616294
; 26756768
; 39289599
; 47439963
; 50111412
; 53787291
; 57357762
; 76781864
; 92126168
; 103591345
; 113853360
; 126523876
; 127302679
; 127302680
; 127302681
; 127302682
; 127302683
; 127302684
; 127302685
; 135651512
; 137005676
; 137240026
; 138355248
; 179293089
; 184584916
; 198947983
; 237822003
; 250135036
; 252455506
; 252467942
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0EW6H
- Formula
- C30H47N3O9S
- Canonical SMILES
- CCCCCC=CCC=CC=CC=CC(C(CCCC(=O)O)O)SCC(C(=O)NCC(=O)O)NC(=O)CCC(C(=O)O)N
- InChI
- 1S/C30H47N3O9S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-25(24(34)15-14-17-27(36)37)43-21-23(29(40)32-20-28(38)39)33-26(35)19-18-22(31)30(41)42/h6-7,9-13,16,22-25,34H,2-5,8,14-15,17-21,31H2,1H3,(H,32,40)(H,33,35)(H,36,37)(H,38,39)(H,41,42)/b7-6-,10-9-,12-11+,16-13+/t22-,23-,24-,25+/m0/s1
- InChIKey
- GWNVDXQDILPJIG-NXOLIXFESA-N
|