| Synonyms |
Uracil riboside; Uracil, 1-beta-D-ribofuranosyl-; DRTQHJPVMGBUCF-XVFCMESISA-N; Uracil-1-beta-d-ribofuranoside; Uridin; WHI7HQ7H85; b-Uridine; beta-Uridine; d-uridine; uridine; uridine-1'-13C; 1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione; 1-.beta.-D-Ribofuranosyluracil; 1-beta-D-Ribofuranosyluracil; 58-96-8; AI3-52690; C9H12N2O6; CHEBI:16704; EINECS 200-407-5; MFCD00006526; MLS000069625; NSC 20256; SMR000058222; UNII-WHI7HQ7H85; Urd
|
| Cross-matching ID |
- PubChem CID
- 6029
- PubChem SID
-
3275
; 3593
; 584045
; 825827
; 3134852
; 3718411
; 7891023
; 8139953
; 8144667
; 8153756
; 12109174
; 14710325
; 14847540
; 15221017
; 24900434
; 24900638
; 24900640
; 26702452
; 26715956
; 26754144
; 29225043
; 46167442
; 46391419
; 46504323
; 48095962
; 49833656
; 50059626
; 56459453
; 57323128
; 77021584
; 81044517
; 81092927
; 85164984
; 85230753
; 87577750
; 88835504
; 92298506
; 99226784
; 99438033
; 103313732
; 103852268
; 104310914
; 117358157
; 117613647
; 118048883
; 123059964
; 124525105
; 124799625
; 124882993
; 126523145
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y7DP
- Formula
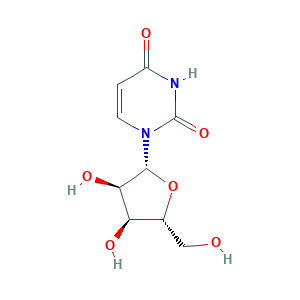
- C9H12N2O6
- Canonical SMILES
- C1=CN(C(=O)NC1=O)C2C(C(C(O2)CO)O)O
- InChI
- 1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1
- InChIKey
- DRTQHJPVMGBUCF-XVFCMESISA-N
|