| General Information of Drug (ID:
DR2272) |
| Drug Name |
Cimetidine
|
| Synonyms |
Cimetadine; Cimetag; Cimetidina; Cimetidina [INN-Spanish]; Cimetidine (JP15/USP/INN); Cimetidine Hcl; Cimetidine [USAN:INN:BAN:JAN]; Cimetidinum; Cimetidinum [INN-Latin]; Cimetum; Dyspamet; Edalene; Eureceptor; Evicer; Gastrobitan; Acibilin; Acinil; Altramet; Biomet400; Brumetidina; CIMETIDINE A/AB; Gastromet; Histodil; Magicul; Metracin; Peptol; SK&F-92334; SKF 92334; SKF-92334; Sigmetadine; Tagamet; Tagamet (TN); Tagamet HB (TN); Tagamet HB200 (TN); Tagamet Hb; Tagamet Hb 200; Tametin; Tratul; Ulcedin; Ulcedine; Ulcestop; Ulcimet; Ulcofalk; Ulcomedina; Ulcomet; Valmagen; Venopex; 1-Cyano-2-methyl-3-(2-(((5-methyl-4-imidazolyl)methyl)thio)ethyl)guanidine; 1-Cyano-2-methyl-3-[2-[[(5-methylimidazol-4-yl)methyl]thio]ethyl]guanidine; 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine; 2-Cyano-1-methyl-3-(2-(((5-methylimidazol-4-yl)methyl)thio)ethyl)guanidine; 2-Cyano-1-methyl-3-[2-(5-methyl-1H-imidazol-4-yl-methylthio)ethyl]guanidine; 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine; 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; C 4522; Ci metum; Cimal; DRG-0150; FPF 1002; N''-Cyano-N-methyl-N'-[2-[(5-methyl-1H-imidazol-4-yl)methylthio]ethyl]guanidine; N''-cyano-N-methyl-N'-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)-ethyl)guanidine; N''-cyano-N-methyl-N'-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; N-Cyano-N'-Methyl-N''-(2-(((5-Methyl-1H-Imidazol-4-YL)Methyl)Thio)Ethyl) Guanidine; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1 H-imidazol-4-yl) methyl)thio)ethyl)guanidine; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)guanidine; N-Cyano-N'-methyl-[2-[[[5-methyl-1H-imidazol-4-yl]methyl]thio]ethyl]guanidine; N-cyano-N'-methyl-N''-(2-([(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl)ethyl)guanidine; N-cyano-N'-methyl-N''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; Tagamet, SKF-92334, Tratul, Tametin, Dyspamet, Acinil, Cimetidine; Ulhys
|
| Indication |
Duodenal ulcer
[ICD11: DA63]
|
Approved
|
[1]
|
| Structure |
|
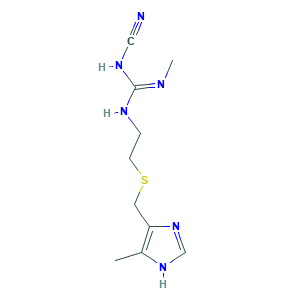 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
252.34 |
Topological Polar Surface Area |
114 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 2756
- PubChem SID
-
9167
; 460110
; 615111
; 4266417
; 4493559
; 6699439
; 6897918
; 7847361
; 7978946
; 8147032
; 8149633
; 10321166
; 10524846
; 11110948
; 11113773
; 11335341
; 11360580
; 11363389
; 11365951
; 11368513
; 11372297
; 11374462
; 11376675
; 11407328
; 11461552
; 11484620
; 11488598
; 11491204
; 11492627
; 11494309
; 11533056
; 12013445
; 14774387
; 15122273
; 15196790
; 17389962
; 17404856
; 22391437
; 24277766
; 24531048
; 26612025
; 26679798
; 26747359
; 26747360
; 26751956
; 26751957
; 29221911
; 46487919
; 46505360
; 47275096
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02DPA
- Formula
- C10H16N6S
- Canonical SMILES
- CC1=C(N=CN1)CSCCNC(=NC)NC#N
- InChI
- 1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14)
- InChIKey
- AQIXAKUUQRKLND-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


