| Synonyms |
Pergolida; Pergolide (mesylate); Pergolide Methanesulfonate; Pergolide mesilate; Permax (TN); LY127809; PERGOLIDE MESYLATE; PERGOLIDE MESYLATE SALT; 55B9HQY616; 66104-23-2; 8-beta-((Methylthio)methyl)-6-propylergoline methanesulfonate; 8-beta-((Methylthio)methyl)-6-propylergoline monomethane sulfonate; 8beta-((Methylthio)methyl)-6-propylergoline monomethanesulfonate; CHEBI:8021; CHEMBL1275; CPD000058504; DSSTox_CID_20583; DSSTox_GSID_40583; DSSTox_RID_77029; MLS000069837; MPE; SMR000058504; UNII-55B9HQY616; LY-127,809; LY-127809; Pergolida [INN-Spanish]; Pergolide (INN); Pergolide [INN:BAN]; Pergolidum; Pergolidum [INN-Latin]; Permax; Prestwick0_000295; Prestwick1_000295; Prestwick2_000295; Prestwick3_000295; SR-01000721840; Spectrum2_001970; Spectrum4_000835; Spectrum5_001649; Spectrum_001647; TNP00315; pergolide; (8beta)-8-[(methylsulfanyl)methyl]-6-propylergoline; 24MJ822NZ9; 66104-22-1; CHEBI:63617; CHEMBL531; Ergoline, 8-((methylthio)methyl)-6-propyl-, (8beta)-; NCGC00017366-04; UNII-24MJ822NZ9
|
| Cross-matching ID |
- PubChem CID
- 47812
- ChEBI ID
-
- CAS Number
-
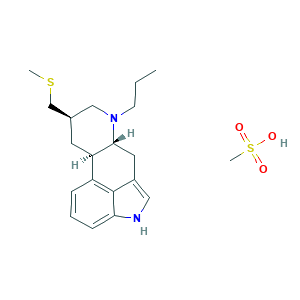
- Formula
- C20H30N2O3S2
- Canonical SMILES
- CCCN1CC(CC2C1CC3=CNC4=CC=CC2=C34)CSC.CS(=O)(=O)O
- InChI
- 1S/C19H26N2S.CH4O3S/c1-3-7-21-11-13(12-22-2)8-16-15-5-4-6-17-19(15)14(10-20-17)9-18(16)21;1-5(2,3)4/h4-6,10,13,16,18,20H,3,7-9,11-12H2,1-2H3;1H3,(H,2,3,4)/t13-,16-,18-;/m1./s1
- InChIKey
- UWCVGPLTGZWHGS-ZORIOUSZSA-N
|