| Synonyms |
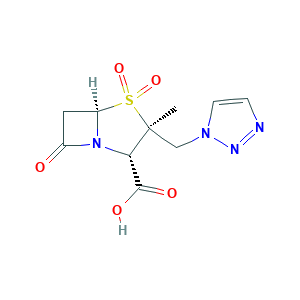
Tazobactam (JAN/USAN/INN); YTR-830H; YTR830H; Piprataz (TN); (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4; (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide; CL-298741; CL298741; TAZ
|
| Cross-matching ID |
- PubChem CID
- 123630
- PubChem SID
-
9973
; 584542
; 604755
; 7847726
; 7890688
; 7980730
; 10240806
; 14751709
; 16012340
; 29303858
; 46508088
; 48416593
; 50192512
; 90444495
; 92711477
; 93166370
; 103163870
; 103949887
; 104418948
; 126630815
; 126657306
; 131329684
; 134338202
; 135693792
; 135841765
; 137248595
; 137981849
; 152100220
; 160964843
; 162009787
; 164196449
; 175267759
; 175607558
; 177749299
; 179316265
; 184544899
; 184816524
; 196106439
; 198993457
; 223438269
; 223554784
; 223684636
; 223703155
; 224294474
; 226493626
; 241152814
; 249868384
; 251912318
; 251916632
; 252074045
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0QQ7D
- Formula
- C10H12N4O5S
- Canonical SMILES
- CC1(C(N2C(S1(=O)=O)CC2=O)C(=O)O)CN3C=CN=N3
- InChI
- 1S/C10H12N4O5S/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19/h2-3,7-8H,4-5H2,1H3,(H,16,17)/t7-,8+,10+/m1/s1
- InChIKey
- LPQZKKCYTLCDGQ-WEDXCCLWSA-N
|