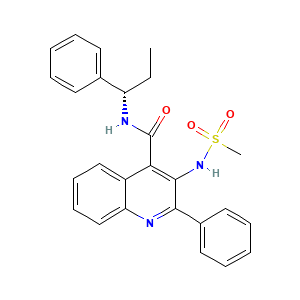
| References |
| 1 |
ClinicalTrials.gov (NCT00686998) A Phase IIa, Double-blind, Double-Dummy, Placebo-controlled, Randomized, Parallel-Group Study to Assess the Efficacy, Safety, Tolerability, and Pharmacokinetics of AZD2624 in Adult Schizophrenia Patients in AstraZeneca.
|
| 2 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038259)
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5775).
|
| 4 |
In vitro assessment of metabolic drugCdrug interaction potential of AZD2624, neurokinin-3 receptor antagonist, through cytochrome P(450) enzyme identification, inhibition, and induction studies
|