| General Information of Drug (ID:
DR3362) |
| Drug Name |
AP26113
|
| Synonyms |
1197958-12-5; ALK-IN-1; UNII-3DGD69C6PV; 3DGD69C6PV; CHEMBL3397300; (2-((5-chloro-2-((4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl)amino)pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide; 5-Chloro-N~2~-{4-[4-(dimethylamino)piperidin-1-yl]-2-methoxyphenyl}-N~4~-[2-(dimethylphosphoryl)phenyl]pyrimidine-2,4-diamine; 5-chloro-2-N-[4-[4-(dimethylamino)piperidin-1-yl]-2-methoxyphenyl]-4-N-(2-dimethylphosphorylphenyl)pyrimidine-2,4-diamine; AP26113-analog; Tube723; compound 11q [PMID: 27144831]
|
| Indication |
Solid tumour/cancer
[ICD11: 2A00-2F9Z]
|
Phase 2
|
[1]
|
| Structure |
|
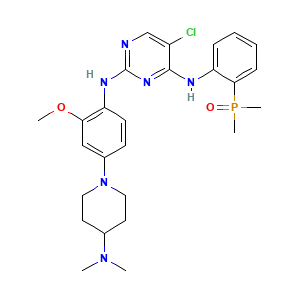 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
529 |
Topological Polar Surface Area |
82.6 |
| Heavy Atom Count |
36 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 57390074
- CAS Number
-
- TTD Drug ID
- D0O9PQ
- Formula
- C26H34ClN6O2P
- Canonical SMILES
- CN(C)C1CCN(CC1)C2=CC(=C(C=C2)NC3=NC=C(C(=N3)NC4=CC=CC=C4P(=O)(C)C)Cl)OC
- InChI
- InChI=1S/C26H34ClN6O2P/c1-32(2)18-12-14-33(15-13-18)19-10-11-21(23(16-19)35-3)30-26-28-17-20(27)25(31-26)29-22-8-6-7-9-24(22)36(4,5)34/h6-11,16-18H,12-15H2,1-5H3,(H2,28,29,30,31)
- InChIKey
- OVDSPTSBIQCAIN-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


