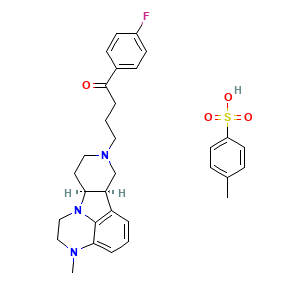
| Synonyms |
lumateperone Tosylate; lumateperone (Tosylate); 1187020-80-9; UNII-JIE88N006O; ITI-007 tosylate; JIE88N006O; 1187020-80-9 (tosylate); ITI007; ITI 007; ITI-007; Lumateperone tosylate (USAN); Lumateperone tosylate [USAN]; CHEMBL3233142; 1-(4-fluorophenyl)-4-[(6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl]butan-1-one toluenesulfonic acid; Caplyta (TN); SCHEMBL1769664; CS-5540; HY-19733; D11170; Q27281520; 1-(4-Fluoro-phenyl)-4-((6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H,7H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8-yl)-butan-1-one tosylate salt
|
| Cross-matching ID |
- PubChem CID
- 44241743
- CAS Number
-
- TTD Drug ID
- D1CWL0
- Formula
- C31H36FN3O4S
- Canonical SMILES
- CC1=CC=C(C=C1)S(=O)(=O)O.CN1CCN2[C@H]3CCN(C[C@H]3C4=C2C1=CC=C4)CCCC(=O)C5=CC=C(C=C5)F
- InChI
- InChI=1S/C24H28FN3O.C7H8O3S/c1-26-14-15-28-21-11-13-27(16-20(21)19-4-2-5-22(26)24(19)28)12-3-6-23(29)17-7-9-18(25)10-8-17;1-6-2-4-7(5-3-6)11(8,9)10/h2,4-5,7-10,20-21H,3,6,11-16H2,1H3;2-5H,1H3,(H,8,9,10)/t20-,21-;/m0./s1
- InChIKey
- LHAPOGAFBLSJJQ-GUTACTQSSA-N
|