Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR5510) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Penicillin G Sodium
|
|||||
| Synonyms |
Penicillin G sodium salt; 69-57-8; Benzylpenicillin sodium; Crystapen; American penicillin; Pencillin G sodium; Sodium penicillin G; BENZYLPENICILLIN SODIUM SALT; Sodium penicillin; Sodium benzylpenicillinate; Sodium penicillin II; Veticillin; Penilaryn; Mycofarm; Novocillin; Sodium benzylpenicillin; Kesso-Pen; Pen-A-Brasive; Sodium benzylpenicillin G; Monosodium benzylpenicillin; Benzylpenicillinic acid sodium salt; Sugracillin sodium salt; Penicillin G Sodium, Crystalline; Sodium benzylp
|
|||||
| Indication | Bacterial infection [ICD11: 1A00-1C4Z] | Approved | [1] | |||
| Structure |
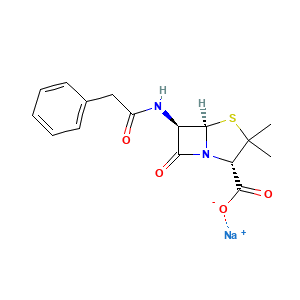 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 356.4 | Topological Polar Surface Area | 115 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| 2 | Enzyme-catalyzed biodegradation of penicillin fermentation residues by -lactamase OtLac from Ochrobactrum tritici | |||||
| 3 | Whole-Cell Biotransformation of Penicillin G by a Three-Enzyme Co-expression System with Engineered Deacetoxycephalosporin?C Synthase | |||||
| 4 | A breakthrough in enzyme technology to fight penicillin resistance-industrial application of penicillin amidase | |||||
| 5 | Acylation and deacylation mechanism and kinetics of penicillin G reaction with Streptomyces R61 DD-peptidase | |||||
| 6 | Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid in man and its significance in assessing penicillin absorption | |||||
| 7 | Identification of new minor metabolites of penicillin G in human serum by multiple-stage tandem mass spectrometry | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

