| Synonyms |
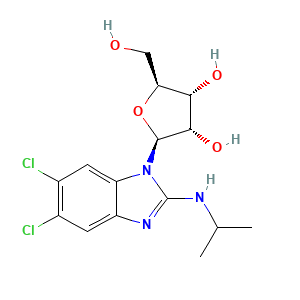
176161-24-3; Benzimidavir; (2S,3S,4R,5S)-2-(5,6-dichloro-2-(isopropylamino)-1H-benzo[d]imidazol-1-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol; 1263W94; UNII-PTB4X93HE1; PTB4X93HE1; 5,6-Dichloro-2-(isopropylamino)-1-(beta-L-ribofuranosyl)-1H-benzimidazole; 5,6-Dichloro-N-(1-methylethyl)-1-beta-L-ribofuranosyl-1H-benzimidazol-2-amine; (2S,3S,4R,5S)-2-[5,6-dichloro-2-(propan-2-ylamino)benzimidazol-1-yl]-5-(hydroxymethyl)oxolane-3,4-diol; Maribavir [USAN:INN:BAN]; Camvia; C15H19Cl2N3O4; Camvia(TM); Camvia (TN); Bzurea
|
| Cross-matching ID |
- PubChem CID
- 471161
- CAS Number
-
- TTD Drug ID
- D07WXI
- Formula
- C15H19Cl2N3O4
- Canonical SMILES
- CC(C)NC1=NC2=CC(=C(C=C2N1[C@@H]3[C@H]([C@H]([C@@H](O3)CO)O)O)Cl)Cl
- InChI
- InChI=1S/C15H19Cl2N3O4/c1-6(2)18-15-19-9-3-7(16)8(17)4-10(9)20(15)14-13(23)12(22)11(5-21)24-14/h3-4,6,11-14,21-23H,5H2,1-2H3,(H,18,19)/t11-,12-,13-,14-/m0/s1
- InChIKey
- KJFBVJALEQWJBS-XUXIUFHCSA-N
|