| Synonyms |
ALDOSTERONE; Aldocorten; Aldocortene; Aldocortin; Aldosterona; Aldosterona [INN-Spanish]; Aldosterone [INN:BAN:DCF]; Aldosteronum; Aldosteronum [INN-Latin]; Electrocortin; Elektrocortin; Reichstein X; d-Aldosterone; (+)-Aldosterone; 11beta,21-Dihydroxy-3,20-diketo-4-pregnen-18-al; 11beta,21-Dihydroxy-3,20-diketopregn-4-ene-18-al; 11beta,21-Dihydroxypregn-4-ene-3,18,20-trione; 18-Aldocorticosterone; 18-Formyl-11beta,21-dihydroxy-4-pregnene-3,20-dione; 18-Oxocorticosterone; 52-39-1; NSC 73856; UNII-4964P6T9RB; [3H]aldosterone
|
| Cross-matching ID |
- PubChem CID
- 5839
- PubChem SID
-
4910
; 115922
; 625873
; 841789
; 3139122
; 7885975
; 7978658
; 8144488
; 8153606
; 12146619
; 14828271
; 24702310
; 24891458
; 26757653
; 29224871
; 46505770
; 48415527
; 48421925
; 50019585
; 53789139
; 56310979
; 56311296
; 56311417
; 56312971
; 56313892
; 56314372
; 56320629
; 56320630
; 57322992
; 57392512
; 77125022
; 85845809
; 87219227
; 93165963
; 103174056
; 104310406
; 123120918
; 124800418
; 126666510
; 128954713
; 134338558
; 134972910
; 135651432
; 137048176
; 141345016
; 160967623
; 163076031
; 172097284
; 175268093
; 178100450
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0I1LH
- Formula
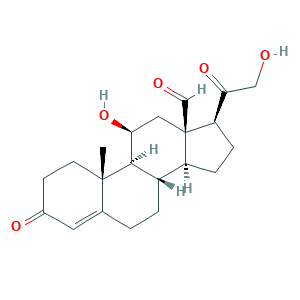
- C21H28O5
- Canonical SMILES
- CC12CCC(=O)C=C1CCC3C2C(CC4(C3CCC4C(=O)CO)C=O)O
- InChI
- 1S/C21H28O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h8,11,14-17,19,22,25H,2-7,9-10H2,1H3/t14-,15-,16+,17-,19+,20-,21+/m0/s1
- InChIKey
- PQSUYGKTWSAVDQ-ZVIOFETBSA-N
|