| Synonyms |
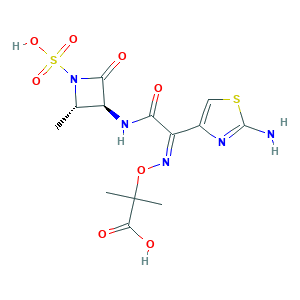
Azactam; Azthreonam; Corus 1020; Primbactam; SQ 26776; SQ-26776; Squibb 26776; aztreonam; 2-[[(Z)-[1-(2-Amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methylpropionic Acid; 78110-38-0; CHEBI:161680; CHEMBL158; G2B4VE5GH8; MFCD00072145; NSC646279; UNII-G2B4VE5GH8; [2S-[2alpha,3beta(Z)]]-2-[[[1-(2-Amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic acid
|
| Cross-matching ID |
- PubChem CID
- 5742832
- PubChem SID
-
503035
; 605760
; 7978752
; 8142308
; 10321853
; 11765528
; 12013309
; 14783375
; 14930214
; 39538698
; 47871110
; 48020413
; 48244800
; 48395280
; 48415600
; 49698380
; 50124378
; 50768269
; 57365010
; 85787954
; 91652037
; 92125075
; 92309205
; 92713785
; 93166923
; 103220466
; 103914334
; 104098313
; 104253180
; 114107021
; 119526244
; 121362746
; 124757300
; 125164104
; 126624596
; 126655874
; 135692167
; 136368064
; 136949096
; 139813750
; 143491685
; 144115675
; 151979576
; 152101196
; 152242936
; 160871057
; 162037635
; 164787753
; 170496669
; 174549401
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0F2XV
- Formula
- C13H17N5O8S2
- Canonical SMILES
- CC1C(C(=O)N1S(=O)(=O)O)NC(=O)C(=NOC(C)(C)C(=O)O)C2=CSC(=N2)N
- InChI
- 1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1
- InChIKey
- WZPBZJONDBGPKJ-VEHQQRBSSA-N
|