| General Information of Drug (ID:
DR0177) |
| Drug Name |
Bazedoxifene
|
| Synonyms |
Bazedoxifene; Bazedoxifene [INN]; Bazedoxifeno; Bazedoxifeno [INN-Spanish]; Conbriza; Q16TT9C5BK; TSE-424; WAY 140424; 1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol; 1-[4-(2-Azepan-1-yl-ethoxy)-benzyl]-2-(4-hydroxy-phenyl)-3-methyl-1H-indol-5-ol; 1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methylindol-5-ol; 198481-32-2; 1H-Indol-5-ol, 1-[[4-[2-(hexahydro-1H-azepin-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-; C30H34N2O3; CHEMBL46740; UNII-Q16TT9C5BK
|
| Indication |
Schizophrenia
[ICD11: 6A20]
|
Phase 4
|
[1]
|
| Structure |
|
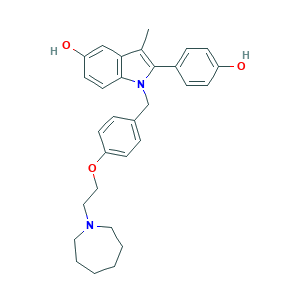 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
470.6 |
Topological Polar Surface Area |
57.9 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 154257
- PubChem SID
-
5417414
; 10252039
; 14907497
; 46230413
; 50409595
; 103224092
; 103996451
; 104438324
; 125669539
; 129348176
; 135129358
; 137115372
; 142033963
; 160647805
; 162810855
; 163621139
; 175267431
; 178103927
; 179150132
; 185964861
; 198992700
; 204379955
; 223780042
; 226426848
; 251971047
; 252434487
; 252477814
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0JY8T
- Formula
- C30H34N2O3
- Canonical SMILES
- CC1=C(N(C2=C1C=C(C=C2)O)CC3=CC=C(C=C3)OCCN4CCCCCC4)C5=CC=C(C=C5)O
- InChI
- 1S/C30H34N2O3/c1-22-28-20-26(34)12-15-29(28)32(30(22)24-8-10-25(33)11-9-24)21-23-6-13-27(14-7-23)35-19-18-31-16-4-2-3-5-17-31/h6-15,20,33-34H,2-5,16-19,21H2,1H3
- InChIKey
- UCJGJABZCDBEDK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


