| Synonyms |
Ciclesonide; Ciclesonide [INN]; Omnair; Omnaris; Omnaris HFA; Alvesco; Alvesco HFA; Osonase; Osonide; RPR 251526; RPR-251526; RPR251526; S59502J185; Zetonna; (R)-11beta,16alpha,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with cyclohexanecarboxaldehyde, 21-isobutyrate; 126544-47-6; 141845-82-1; AC1MIWNR; Pregna-1,4-diene-3,20-dione, 16,17-((cyclohexylmethylene)bis(oxy))-11-hydroxy-21-(2-methyl-1-oxopropoxy)-, (11beta,16alpha(R))-; UNII-S59502J185
|
| Cross-matching ID |
- PubChem CID
- 6918155
- PubChem SID
-
7848766
; 12014485
; 14763472
; 14910303
; 17194708
; 43529548
; 50069804
; 50113016
; 53790514
; 57371818
; 75476862
; 92719025
; 93307925
; 93815124
; 126592968
; 126621149
; 126652709
; 135211289
; 135805250
; 137002365
; 137619495
; 144206039
; 152134121
; 160668474
; 162183036
; 162258877
; 164788141
; 175266964
; 184545966
; 187051772
; 187072300
; 196106072
; 210279779
; 210282102
; 223657022
; 224423934
; 226395815
; 251915931
; 251917279
; 252215142
; 252347327
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K7HU
- Formula
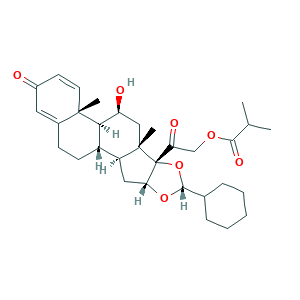
- C32H44O7
- Canonical SMILES
- CC(C)C(=O)OCC(=O)C12C(CC3C1(CC(C4C3CCC5=CC(=O)C=CC45C)O)C)OC(O2)C6CCCCC6
- InChI
- 1S/C32H44O7/c1-18(2)28(36)37-17-25(35)32-26(38-29(39-32)19-8-6-5-7-9-19)15-23-22-11-10-20-14-21(33)12-13-30(20,3)27(22)24(34)16-31(23,32)4/h12-14,18-19,22-24,26-27,29,34H,5-11,15-17H2,1-4H3/t22-,23-,24-,26+,27+,29+,30-,31-,32+/m0/s1
- InChIKey
- LUKZNWIVRBCLON-GXOBDPJESA-N
|