Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0414) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Dapoxetine
|
|||||
| Synonyms |
Dapoxetina; Dapoxetina [INN-Spanish]; Dapoxetine; Dapoxetine [INN]; Dapoxetine hydrochloride; Dapoxetinum; Dapoxetinum [INN-Latin]; GB2433A4M3; Kutub, Priligy, Duratia; LY 210448; LY-210448; LY210448; (+)-Dapoxetine; (1S)-N,N-dimethyl-3-naphthalen-1-yloxy-1-phenylpropan-1-amine; (1s)-n,n-dimethyl-3-(1-naphthyloxy)-1-phenylpropan-1-amine; (S)-N,N-Dimethyl-3-(naphthalen-1-yloxy)-1-phenylpropan-1-amine; 119356-77-3; Benzenemethanamine, N,N-dimethyl-alpha-(2-(1-naphthalenyloxy)ethyl)-, (+)-; DSSTox_RID_97302; UNII-GB2433A4M3
|
|||||
| Indication | Ejaculatory dysfunction [ICD11: HA03] | Phase 4 | [1] | |||
| Structure |
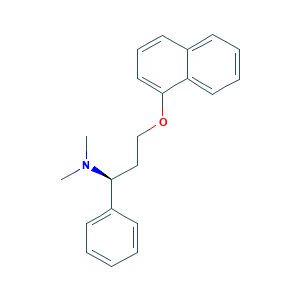 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 305.4 | Topological Polar Surface Area | 12.5 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT01273545) PRILIGY Usage Patterns in Selected Populations. | |||||
| 2 | Dapoxetine: a new option in the medical management of premature ejaculation. Ther Adv Urol. 2012 Oct;4(5):233-51. | |||||
| 3 | Pharmacokinetics of single and multiple escalating doses of dapoxetine in healthy volunteers. Clinical Pharmacology Therapeutics, 2004, 75(2):P32. | |||||
| 4 | Characterization of Phase I Hepatic Metabolites of Anti-Premature Ejaculation Drug Dapoxetine by UHPLC-ESI-Q-TOF | |||||
| 5 | Effects of Evodiamine on the Pharmacokinetics of Dapoxetine and Its Metabolite Desmethyl Dapoxetine in Rats | |||||
| 6 | Effects of 22 novel CYP2D6 variants found in Chinese population on the metabolism of dapoxetine | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

