Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0494) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Diflunisal
|
|||||
| Synonyms |
Difludol; Diflunisalum; Diflunisalum [INN-Latin]; Diflusinal; Dolisal; Dolobid; Dolobil; Dolobis; Flovacil; Fluniget; Fluodonil; Flustar; MK 647; Adomal; Algobid; Citidol; MK-647; Noaldol; Reuflos; Unisal; diflunisal; 2',4'-Difluoro-4-hydroxy-3-biphenylcarboxylic acid; 2-(Hydroxy)-5-(2,4-difluorophenyl)benzoic acid; 22494-42-4; 5-(2,4-Difluorophenyl)salicylic acid; 5-(2,4-difluorophenyl)-2-hydroxybenzoic acid; 5-[2,4-Difluorophenyl]salicylic acid; UNII-7C546U4DEN; [1,1'-Biphenyl]-3-carboxylic acid, 2',4'-difluoro-4-hydroxy-
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Approved | [1] | |||
| Structure |
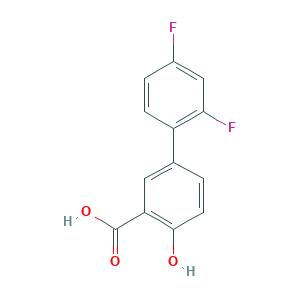 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 250.2 | Topological Polar Surface Area | 57.5 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

