| Synonyms |
AquaGrow Advantage; Cervonic acid; Doconexent; Doconexento; Doconexentum; Docosahexaenoate; Docosahexaenoic acid; Docosahexaenoic acid (all-Z); Doxonexent; Martek DHA HM; Ropufa 60; ZAD9OKH9JC; all-Z-Docosahexaenoic acid; (4Z,7Z,10Z,13Z,16Z,19Z)-Docosahexaenoic acid; (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid; 6217-54-5; CCRIS 7670; CHEMBL367149; UNII-ZAD9OKH9JC; all-cis-4,7,10,13,16,19-Docosahexaenoic acid; all-cis-DHA; all-cis-docosa-4,7,10,13,16,19-hexaenoic acid; cis-4,7,10,13,16,19-Docosahexaenoic acid
|
| Cross-matching ID |
- PubChem CID
- 445580
- PubChem SID
-
8664
; 839059
; 841847
; 7850049
; 8144392
; 10299728
; 14924254
; 24893696
; 26754926
; 26754927
; 26754928
; 36888254
; 46391883
; 47515089
; 47515090
; 47885165
; 48184752
; 50110834
; 50110835
; 57404690
; 75281905
; 81063745
; 85787686
; 87568664
; 91695933
; 92165132
; 92298371
; 92309714
; 92719165
; 99300628
; 99302146
; 103445456
; 104046523
; 104086818
; 104179148
; 104634620
; 118048648
; 126524415
; 126621028
; 126655302
; 134350834
; 135028589
; 135651477
; 137003172
; 142339426
; 144205545
; 152101578
; 162180738
; 162227670
; 163659264
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Q5XX
- Formula
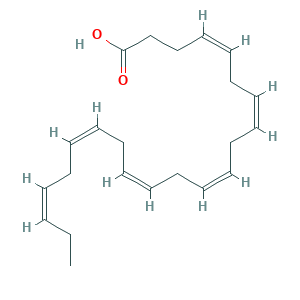
- C22H32O2
- Canonical SMILES
- CCC=CCC=CCC=CCC=CCC=CCC=CCCC(=O)O
- InChI
- 1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-
- InChIKey
- MBMBGCFOFBJSGT-KUBAVDMBSA-N
|