| Synonyms |
Ergam; Ergate; Ergotamin; Ergotamina; Ergotamine; Ergotamina [INN-Spanish]; Ergotamine [INN:BAN]; Ergotaminum [INN-Latin]; Ergoton-A; HSDB 4076; Medihaler ergotamine; NSC 95090; PR834Q503T; Temigran; UNII-PR834Q503T; Wigrettes; Ergotaminum; 113-15-5; 12'-Hydroxy-2'-methyl-5'alpha-(phenylmethyl)ergotaman-3',6',18-trione; BRN 0078890; CHEBI:64318; Cornutamine; EINECS 204-023-9; ERGOTAMINE; Ergonsvine; Ergostat; Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(phenylmethyl)-, (5'alpha)-; Gynergin; I07-0341; LS-177242; Lingraine; Lingran; NSC 41869; Neo-ergotin; SCHEMBL8045; Secagyn; Secupan; (5; 379-79-3; AC-14836; AKOS015897172; C33H35N5O5.C4H6O6; CHEMBL2062265
|
| Cross-matching ID |
- PubChem CID
- 16051995
- CAS Number
-
- TTD Drug ID
- D01TSI
- Formula
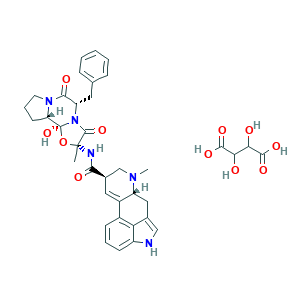
- C37H41N5O11
- Canonical SMILES
- CC1(C(=O)N2C(C(=O)N3CCCC3C2(O1)O)CC4=CC=CC=C4)NC(=O)C5CN(C6CC7=CNC8=CC=CC(=C78)C6=C5)C.C(C(C(=O)O)O)(C(=O)O)O
- InChI
- 1S/C33H35N5O5.C4H6O6/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32;5-1(3(7)8)2(6)4(9)10/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39);1-2,5-6H,(H,7,8)(H,9,10)/t21-,25-,26+,27+,32-,33+;/m1./s1
- InChIKey
- NMTWKEWYQXZGCI-DDLCCZDQSA-N
|