| General Information of Drug (ID:
DR0839) |
| Drug Name |
Hydroxyprogesterone caproate
|
| Synonyms |
Hydroxyprogesteronum; Hydroxyprogesteronum [INN-Latin]; Idrossiprogesterone [DCIT]; Oxiprogesteronum; Prodix; Prodox; Setaderm; hydroxyprogesterone; 17-Hydroxypregn-4-ene-3,20-dione; 17-Hydroxyprogesterone; 17-alpha-Hydroxyprogesterone; 17ALPHA-HYDROXYPROGESTERONE; 17a-Hydroxyprogesterone; 17alpha-Hydroxy-4-pregnene-3,20-dione; 17alpha-Hydroxy-progesterone; 68-96-2; Pregn-4-ene-3,20-dione, 17-hydroxy-; delta(4)-Pregnene-17alpha-ol-3,20-dione; Gestageno; Gestageno gador; Hidroxiprogesterona; Hidroxiprogesterona [INN-Spanish]; A-Hydroxyprogesterone caproate; Corlutin L.A.; Delalutin; Depo-Proluton; Duraluton; Estralutin; Gesterol LA 250; HYDROXYPROGESTERONE CAPROATE; Hormofort; Hydroxyprogesterone hexanoate; Hylutin; Hyproval; Hyproval-PA; Hyroxon; Idrogestene; Kaprogest; Lutate; Luteocrin; Luteocrin depot; Lutopron; Neolutin; Neolutin forte; Primolut Depot; Progesterone caproate; Progesterone retard pharlon; Proluton Depot; Relutin; Syngynon; Teralutil; 17alpha-Caproyloxypregn-4-ene-3,20-dione; 17alpha-Hydroxyprogesterone caproate; 630-56-8; Proge
|
| Indication |
Uterine cancer
[ICD11: 2C76]
|
Approved
|
[1]
|
| Structure |
|
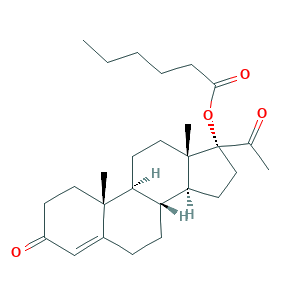 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
428.6 |
Topological Polar Surface Area |
60.4 |
| Heavy Atom Count |
31 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 169870
- PubChem SID
-
10348
; 80880
; 855528
; 7848012
; 10257140
; 12209772
; 15007174
; 24895697
; 46243005
; 49834232
; 53788881
; 56424128
; 57351759
; 75144784
; 81093219
; 87571349
; 90473603
; 93576739
; 103770831
; 103823738
; 113466682
; 117622268
; 124576771
; 124799532
; 124886752
; 127764370
; 131321883
; 131549786
; 134976956
; 137124474
; 143449668
; 144207051
; 144212650
; 160843844
; 162092117
; 163270260
; 164788675
; 170465210
; 174529979
; 175265862
; 179316277
; 184545682
; 198993584
; 223439966
; 226397056
; 241113174
; 250112560
; 252122136
; 252308498
; 252401932
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00AEQ
- Formula
- C27H40O4
- Canonical SMILES
- CCCCCC(=O)OC1(CCC2C1(CCC3C2CCC4=CC(=O)CCC34C)C)C(=O)C
- InChI
- 1S/C27H40O4/c1-5-6-7-8-24(30)31-27(18(2)28)16-13-23-21-10-9-19-17-20(29)11-14-25(19,3)22(21)12-15-26(23,27)4/h17,21-23H,5-16H2,1-4H3/t21-,22+,23+,25+,26+,27+/m1/s1
- InChIKey
- DOMWKUIIPQCAJU-LJHIYBGHSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


