| General Information of Drug (ID:
DR0856) |
| Drug Name |
Iloperidone
|
| Synonyms |
Iloperidone; Iloperidone (Fanapt); Iloperidone(Fanapt); VPO7KJ050N; Zomaril; 1-(4-(3-(4-(6-Fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)propoxy)-3-methoxyphenyl)ethanone; 1-[4-[3-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]propoxy]-3-methoxyphenyl]ethanone; Fanapt; Fanapta; HP 873; HP-873; 133454-47-4; 4'-(3-(4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino)propoxy)-3'-methoxyacetophenone; C24H27FN2O4; CHEBI:65173; CHEMBL14376; DSSTox_CID_28986; DSSTox_GSID_49060; DSSTox_RID_83251; NCGC00188864-01; UNII-VPO7KJ050N
|
| Indication |
Schizophrenia
[ICD11: 6A20]
|
Approved
|
[1]
|
| Structure |
|
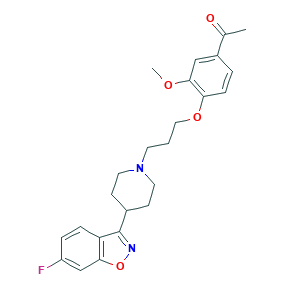 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
426.5 |
Topological Polar Surface Area |
64.8 |
| Heavy Atom Count |
31 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 71360
- PubChem SID
-
8194581
; 12014542
; 14905142
; 17396835
; 43127698
; 47359860
; 47359861
; 47731199
; 48404440
; 50064358
; 51401239
; 57318156
; 85209859
; 92712438
; 93301492
; 99246148
; 99437181
; 103176655
; 103856472
; 103942259
; 104350273
; 109693466
; 117566372
; 118050578
; 124757285
; 124899318
; 125164089
; 125340171
; 126625565
; 126656709
; 126666080
; 129711844
; 131297754
; 134339308
; 135029478
; 135587716
; 135650377
; 135692163
; 135697544
; 136367918
; 137249342
; 142584651
; 144116064
; 144207159
; 152344064
; 160645064
; 162011651
; 162037626
; 162172136
; 163123672
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M8VE
- Formula
- C24H27FN2O4
- Canonical SMILES
- CC(=O)C1=CC(=C(C=C1)OCCCN2CCC(CC2)C3=NOC4=C3C=CC(=C4)F)OC
- InChI
- 1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3
- InChIKey
- XMXHEBAFVSFQEX-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


