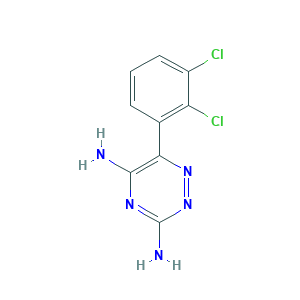
| Synonyms |
Lamictal; Lamictal Cd; Lamictal ODT; Lamictal XR; Lamotrigina; Lamotrigina [Spanish]; Lamotrigine, 98%; Lamotriginum; Lamotriginum [Latin]; PYZRQGJRPPTADH-UHFFFAOYSA-N; U3H27498KS; lamotrigine; 1,2,4-Triazine-3,5-diamine, 6-(2,3-dichlorophenyl)-; 3,5-Diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine; 3,5-Diamino-6-(2,3-dichlorophenyl)-as-triazine; 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine; 84057-84-1; BW 430C; BW-430C; C9H7Cl2N5; CHEBI:6367; CHEMBL741; EINECS 281-901-8; GW 273293; MFCD00865333; MLS000069685; UNII-U3H27498KS
|
| Cross-matching ID |
- PubChem CID
- 3878
- PubChem SID
-
855818
; 5669156
; 7847420
; 7979720
; 8152444
; 11111377
; 11113342
; 11528749
; 12013463
; 14823602
; 17405249
; 24278792
; 26719658
; 29222992
; 46386680
; 46505408
; 47810853
; 48259345
; 48416154
; 50100261
; 50104089
; 50104090
; 53777794
; 53787568
; 56423136
; 57322032
; 58107296
; 76843584
; 81092819
; 85231112
; 90341162
; 91148150
; 92304062
; 92308151
; 92308280
; 92308924
; 92709766
; 93166952
; 103199382
; 103940000
; 104179107
; 104304778
; 111978157
; 117872151
; 121361621
; 124658861
; 124799528
; 124880544
; 124880545
; 124880546
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03FLC
- Formula
- C9H7Cl2N5
- Canonical SMILES
- C1=CC(=C(C(=C1)Cl)Cl)C2=C(N=C(N=N2)N)N
- InChI
- 1S/C9H7Cl2N5/c10-5-3-1-2-4(6(5)11)7-8(12)14-9(13)16-15-7/h1-3H,(H4,12,13,14,16)
- InChIKey
- PYZRQGJRPPTADH-UHFFFAOYSA-N
|