| DM Name |
DM ID |
PubChem ID |
Reaction |
DM Level |
REF |
|
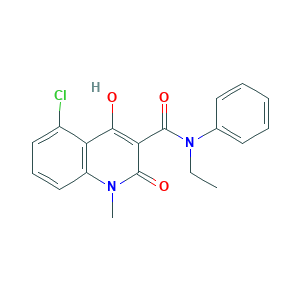
5-chloro-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide
|
DM006019
|
|
Oxidation
-
N-dealkylation |
1 |
[3]
|
|
5-chloro-N-ethyl-4,6-dihydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide
|
DM006023
|
|
Oxidation
-
Aromatic hydroxylation |
1 |
[3]
|
|
5-chloro-N-ethyl-4,7-dihydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide
|
DM006022
|
|
Oxidation
-
Aromatic hydroxylation |
1 |
[3]
|
|
5-chloro-N-ethyl-4,8-dihydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide
|
DM006021
|
|
Oxidation
-
Aromatic hydroxylation |
1 |
[3]
|
|
5-chloro-N-ethyl-4-hydroxy-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide
|
DM006020
|
|
Oxidation
-
N-dealkylation |
1 |
[3]
|
|
5-chloro-N-ethyl-4-hydroxy-N-(4-hydroxyphenyl)-1-methyl-2-oxo-1,2-dihydroquinoline-3-carboxamide
|
DM006018
|
|
Oxidation
-
Aromatic hydroxylation |
1 |
[3]
|
|
|
|
|
|
|