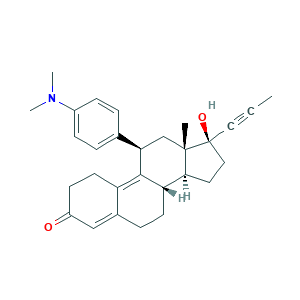
| Cross-matching ID |
- PubChem CID
- 55245
- PubChem SID
-
9854
; 610895
; 827617
; 7847651
; 7885329
; 7979988
; 8184037
; 10321543
; 11466327
; 11467447
; 11486193
; 11533034
; 12013919
; 12146248
; 14758749
; 14905326
; 17405362
; 24278572
; 26752199
; 26759172
; 34719152
; 46386955
; 46505795
; 46518693
; 47573358
; 47720589
; 47795017
; 47795018
; 48018884
; 48318385
; 48393908
; 48416281
; 49698423
; 49835339
; 49965772
; 50124354
; 50576278
; 53777907
; 53788556
; 56312231
; 56313355
; 56313657
; 56313765
; 56352854
; 56424093
; 57313727
; 85752924
; 85789352
; 90341299
; 92125204
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Z4EI
- Formula
- C29H35NO2
- Canonical SMILES
- CC#CC1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O
- InChI
- 1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1
- InChIKey
- VKHAHZOOUSRJNA-GCNJZUOMSA-N
|