| General Information of Drug (ID:
DR1361) |
| Drug Name |
Propafenone hydrochloride
|
| Synonyms |
Propafenon hydrochlorid; Propafenon hydrochlorid [German]; Propafenone (hydrochloride); Propafenone HCl; Rhythmonorm; Rythmol SR; Rytmonorm; WZ 884; 1-(2-(2-Hydroxy-3-(propylamino)propoxy)phenyl)-3-phenylpropan-1-one hydrochloride; 1-[2-[2-hydroxy-3-(propylamino)propoxy]phenyl]-3-phenylpropan-1-one hydrochloride; 1-{2-[2-hydroxy-3-(propylamino)propoxy]phenyl}-3-phenylpropan-1-one hydrochloride; 34183-22-7; EINECS 251-867-9; MLS000069682; SA 79; Arythmol; Baxarytmon; Fenoprain; PROPAFENONE HYDROCHLORIDE; Pronon
|
| Indication |
Ventricular tachyarrhythmia
[ICD11: BC71]
|
Approved
|
[1]
|
| Structure |
|
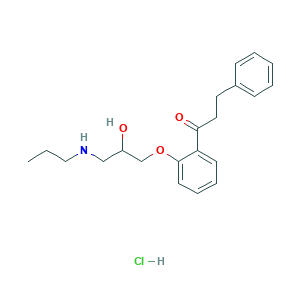 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
377.9 |
Topological Polar Surface Area |
58.6 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 36708
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J2KV
- Formula
- C21H28ClNO3
- Canonical SMILES
- CCCNCC(COC1=CC=CC=C1C(=O)CCC2=CC=CC=C2)O.Cl
- InChI
- 1S/C21H27NO3.ClH/c1-2-14-22-15-18(23)16-25-21-11-7-6-10-19(21)20(24)13-12-17-8-4-3-5-9-17;/h3-11,18,22-23H,2,12-16H2,1H3;1H
- InChIKey
- XWIHRGFIPXWGEF-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


