| DM Name |
DM ID |
PubChem ID |
Reaction |
DM Level |
REF |
|
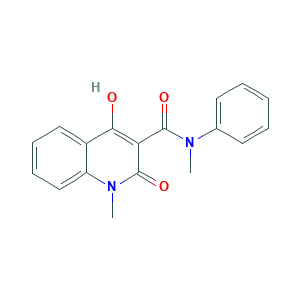
4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide
|
DM005601
|
|
Unclear |
1 |
[2]
,
[3]
|
|
PNU-212679 N-Phenyl-N-methyl-2-oxo-4-hydroxy-1,2-dihydroquinoline-3-carboxamide
|
DM005602
|
|
Unclear |
1 |
[2]
,
[3]
|
|
PNU-212684 4-hydroxy-N-(4-hydroxyphenyl)-N,1-dimethyl-2-oxoquinoline-3-carboxamide
|
DM005600
|
|
Unclear |
1 |
[2]
,
[3]
|
|
PNU-213708 4,6-dihydroxy-N,1-dimethyl-2-oxo-N-phenylquinoline-3-carboxamide
|
DM005597
|
|
Unclear |
1 |
[2]
,
[3]
|
|
PNU-213831 4,8-dihydroxy-N,1-dimethyl-2-oxo-N-phenylquinoline-3-carboxamide
|
DM005599
|
|
Unclear |
1 |
[2]
,
[3]
|
|
PNU-214608 4,7-dihydroxy-N,1-dimethyl-2-oxo-N-phenylquinoline-3-carboxamide
|
DM005598
|
|
Unclear |
1 |
[2]
,
[3]
|
|
R=glucuronic acid OH or sulphate
|
DM005603
|
N. A. |
Unclear |
1 |
[2]
,
[3]
|
|
|
|
|
|
|
|