| Synonyms |
Sibutramina; Sibutramina [Spanish]; Sibutramine [INN:BAN]; Sibutramine hydrochloride; Sibutraminum; Sibutraminum [Latin]; UNAANXDKBXWMLN-UHFFFAOYSA-N; Butramin; Butramin (TN); Medaria; sibutramine; 1-(1-(4-chlorophenyl)cyclobutyl)-N,N,3-trimethylbutan-1-amine; 1-[1-(4-chlorophenyl)cyclobutyl]-N,N,3-trimethyl-butan-1-amine; 1-[1-(4-chlorophenyl)cyclobutyl]-N,N,3-trimethylbutan-1-amine; 106650-56-0; BTS-54524; CHEBI:9137; Cyclobutanemethanamine, 1-(4-chlorophenyl)-N,N-dimethyl-alpha-(2-methylpropyl)-; DEA No. 1675; HSDB 7209
|
| Cross-matching ID |
- PubChem CID
- 5210
- PubChem SID
-
9456
; 3206268
; 4368412
; 7980597
; 8153202
; 11364918
; 11367480
; 11370042
; 11372056
; 11374791
; 11378212
; 11485348
; 11489502
; 11490824
; 11492951
; 11495775
; 14750864
; 29224267
; 46387014
; 46507740
; 47509538
; 47805058
; 48416537
; 49732001
; 49816816
; 49830262
; 50064120
; 50105677
; 50474598
; 57322655
; 81040718
; 85279508
; 92308675
; 92309273
; 92714514
; 93625048
; 96025198
; 103498411
; 104308577
; 108667174
; 118048829
; 121147434
; 124659142
; 124813324
; 125311920
; 125325628
; 125337324
; 126629463
; 126655363
; 126682940
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08KVZ
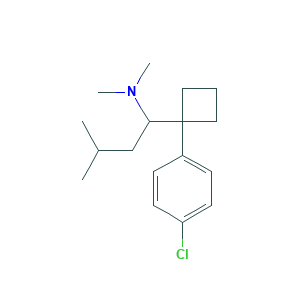
- Formula
- C17H26ClN
- Canonical SMILES
- CC(C)CC(C1(CCC1)C2=CC=C(C=C2)Cl)N(C)C
- InChI
- 1S/C17H26ClN/c1-13(2)12-16(19(3)4)17(10-5-11-17)14-6-8-15(18)9-7-14/h6-9,13,16H,5,10-12H2,1-4H3
- InChIKey
- UNAANXDKBXWMLN-UHFFFAOYSA-N
|