Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1499) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Sonidegib phosphate
|
|||||
| Synonyms |
Sonidegib phosphate; Sonidegib phosphate (USAN); Sonidegib phosphate [USAN:INN]; UNII-W421AI34UW; W421AI34UW; sonidegib bisphosphate; sonidegib diphosphate; 1218778-77-8; 3888AH; AKOS015994565; AOB87343; BCP11860; C26H26F3N3O3.2H3O4P; CHEBI:90864; CHEMBL3137317; CS-1175; DTXSID90669468; EX-A1556; Erismodegib Diphosphate; FE-0016; HY-16582; KS-00002WSZ; LDE-225 Diphosphate; LDE225 (Diphosphate); LDE225 Diphosphate; NVP-LDE225 Diphosphate; NVP-LDE225 Diphosphate salt; Odomzo (TN); SB16677
|
|||||
| Indication | Basal cell carcinoma [ICD11: 2C32] | Approved | [1] | |||
| Structure |
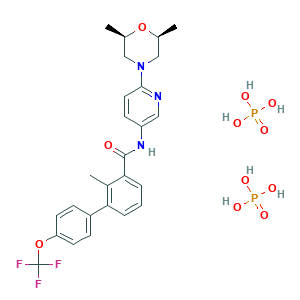 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 681.5 | Topological Polar Surface Area | 219 | ||
| Heavy Atom Count | 45 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 7 | Hydrogen Bond Acceptor Count | 16 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Sonidegib Phosphate was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Sonidegib: first global approval. Drugs. 2015 Sep;75(13):1559-66. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

