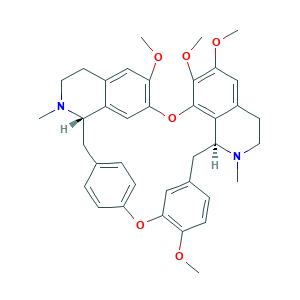
| Synonyms |
D-Tetrandrine; DL-Tetrandine; Fanchinine; Hanfangchin A; S,S-(+)-Tetrandrine; S-(+)-Tetrandrine; Sinomenine A; Tetrandrin; Tetrandrine(Fanchinine); d-Tetrandr; hanjisong; iso-tetrandrine; tetradrine; tetrandrine; (+)-Tetrandrine; (+-)-Tetrandine; (1beta)-6,6',7,12-Tetramethoxy-2,2'-dimethylberbaman; (S,S)-(+)-Tetrandrine; (S,S)-Tetrandrine; 23495-89-8; 29EX23D5AJ; 518-34-3; BRN 0877811; C38H42N2O6; CCRIS 2705; CHEBI:49; MLS002153946; NSC 77037; NSC-77037; NSC77037; NSC91771; SMR000445630; UNII-29EX23D5AJ
|
| Cross-matching ID |
- PubChem CID
- 73078
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G4ES
- Formula
- C38H42N2O6
- Canonical SMILES
- CN1CCC2=CC(=C3C=C2C1CC4=CC=C(C=C4)OC5=C(C=CC(=C5)CC6C7=C(O3)C(=C(C=C7CCN6C)OC)OC)OC)OC
- InChI
- 1S/C38H42N2O6/c1-39-15-13-25-20-32(42-4)34-22-28(25)29(39)17-23-7-10-27(11-8-23)45-33-19-24(9-12-31(33)41-3)18-30-36-26(14-16-40(30)2)21-35(43-5)37(44-6)38(36)46-34/h7-12,19-22,29-30H,13-18H2,1-6H3/t29-,30-/m0/s1
- InChIKey
- WVTKBKWTSCPRNU-KYJUHHDHSA-N
|