| Synonyms |
Tasocitinib citrate; Tofacitinib (CP-690550) Citrate; Tofacitinib (citrate); Tofacitinib citrate; Tofacitinib citrate [USAN]; UNII-O1FF4DIV0D; Xeljanz; Xeljanz Xr; citro; 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile 2-hydroxypropane-1,2,3-tricarboxylate; 540737-29-9; CHEBI:71197; CP 690550 citrate; CP-690,550-10; CP-690550 citrate; CP-690550-10; O1FF4DIV0D
|
| Cross-matching ID |
- PubChem CID
- 10174505
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0EG1I
- Formula
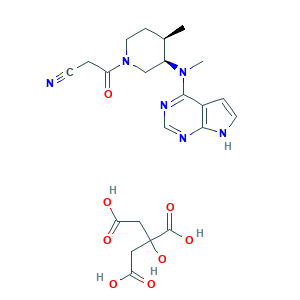
- C22H28N6O8
- Canonical SMILES
- CC1CCN(CC1N(C)C2=NC=NC3=C2C=CN3)C(=O)CC#N.C(C(=O)O)C(CC(=O)O)(C(=O)O)O
- InChI
- 1S/C16H20N6O.C6H8O7/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16;7-3(8)1-6(13,5(11)12)2-4(9)10/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t11-,13+;/m1./s1
- InChIKey
- SYIKUFDOYJFGBQ-YLAFAASESA-N
|