| Synonyms |
Treprostinil; Treprostinil [USAN:INN]; Tresprostinil; Trevyent; Tyvaso; U 62840; U-62,840; Uniprost; treprostinilo; treprostinilum; LRX 15; LRX-15; Orenitram; PAJMKGZZBBTTOY-ZFORQUDYSA-N; RUM6K67ESG; Remodulin; Remodulin (TN); ((1R,2R,3aS,9aS)-2-hydroxy-1-((3S)-3-hydroxyoctyl)-2,3,3a,4,9,9a-hexahydro-1H-cylopent(b)naphthalen-5-yl)oxy)acetate; 15AU81; 2-[[(1R,2R,3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]inden-5-yl]oxy]acetic Acid; 81846-19-7; CHEBI:50861; UNII-RUM6K67ESG; UT-15
|
| Cross-matching ID |
- PubChem CID
- 6918140
- PubChem SID
-
14854155
; 14878554
; 23950807
; 43529534
; 46504572
; 47207871
; 50304326
; 56394875
; 57371812
; 114787499
; 128887520
; 134224636
; 135014308
; 136933659
; 137249659
; 143205120
; 162205084
; 163138465
; 164234753
; 170467450
; 174548947
; 175265596
; 178102445
; 179117156
; 223938513
; 230287018
; 250136886
; 252356445
; 252457273
; 252469194
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01WUA
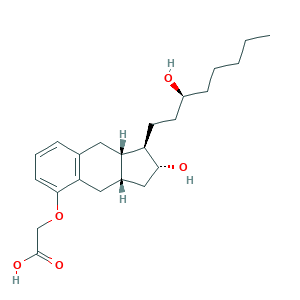
- Formula
- C23H34O5
- Canonical SMILES
- CCCCCC(CCC1C(CC2C1CC3=C(C2)C(=CC=C3)OCC(=O)O)O)O
- InChI
- 1S/C23H34O5/c1-2-3-4-7-17(24)9-10-18-19-11-15-6-5-8-22(28-14-23(26)27)20(15)12-16(19)13-21(18)25/h5-6,8,16-19,21,24-25H,2-4,7,9-14H2,1H3,(H,26,27)/t16-,17-,18+,19-,21+/m0/s1
- InChIKey
- PAJMKGZZBBTTOY-ZFORQUDYSA-N
|