| Synonyms |
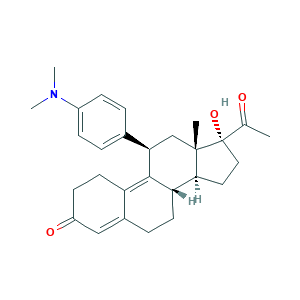
Uliprisnil; Ulipristal; Ulipristal (USAN/INN); Ulipristal [USAN:INN:BAN]; VA-2914; AMX10182; CDB 3236; Deacetyl CDB 2914; HKDLNTKNLJPAIY-WKWWZUSTSA-N; PGL 4001; SCHEMBL545159; (8S,11R,13S,14S,17R)-17-acetyl-11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-6,7,8,11,12,13,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3(2H)-one; 159811-51-5; 6J5J15Q2X8; C28H35NO3; CHEMBL2103846; UNII-6J5J15Q2X8; ellae
|
| Cross-matching ID |
- PubChem CID
- 13559281
- PubChem SID
-
24841789
; 28676416
; 35849341
; 124490307
; 135271930
; 160687014
; 164235293
; 165245594
; 172919799
; 175266288
; 198993968
; 223435395
; 226854508
; 252228535
; 252432863
; 252473760
- CAS Number
-
- TTD Drug ID
- D0V4WD
- Formula
- C28H35NO3
- Canonical SMILES
- CC(=O)C1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O
- InChI
- 1S/C28H35NO3/c1-17(30)28(32)14-13-25-23-11-7-19-15-21(31)10-12-22(19)26(23)24(16-27(25,28)2)18-5-8-20(9-6-18)29(3)4/h5-6,8-9,15,23-25,32H,7,10-14,16H2,1-4H3/t23-,24+,25-,27-,28-/m0/s1
- InChIKey
- HKDLNTKNLJPAIY-WKWWZUSTSA-N
|