| Synonyms |
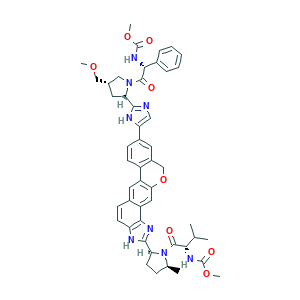
Velpatasvir; Velpatasvir [USAN:INN]; Verpatasvir; KCU0C7RS7Z; 1377049-84-7; GS 5816; GS-5816; GS5816; UNII-KCU0C7RS7Z; methyl {(2S)-1-[(2S,5S)-2-(9-{2-[(2S,4S)-1-{(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetyl}-4-(methoxymethyl)pyrrolidin-2-yl]-1H-imidazol-4-yl}-1,11-dihydro[2]benzopyrano[4',3':6,7]naphtho[1,2-d]imidazol-2-yl)-5-methylpyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl}carbamate
|
| Cross-matching ID |
- PubChem CID
- 67683363
- ChEBI ID
-
- CAS Number
-
- Formula
- C49H54N8O8
- Canonical SMILES
- CC1CCC(N1C(=O)C(C(C)C)NC(=O)OC)C2=NC3=C(N2)C=CC4=CC5=C(C=C43)OCC6=C5C=CC(=C6)C7=CN=C(N7)C8CC(CN8C(=O)C(C9=CC=CC=C9)NC(=O)OC)COC
- InChI
- 1S/C49H54N8O8/c1-26(2)41(54-48(60)63-5)47(59)57-27(3)12-17-38(57)45-51-36-16-14-30-20-35-33-15-13-31(19-32(33)25-65-40(35)21-34(30)43(36)53-45)37-22-50-44(52-37)39-18-28(24-62-4)23-56(39)46(58)42(55-49(61)64-6)29-10-8-7-9-11-29/h7-11,13-16,19-22,26-28,38-39,41-42H,12,17-18,23-25H2,1-6H3,(H,50,52)(H,51,53)(H,54,60)(H,55,61)/t27-,28-,38-,39-,41-,42+/m0/s1
- InChIKey
- FHCUMDQMBHQXKK-CDIODLITSA-N
|