| General Information of Drug (ID:
DR1760) |
| Drug Name |
Dexmethylphenidate
|
| Synonyms |
Dexmethylphenidate; Dexmethylphenidate (INN); Dexmethylphenidate [INN]; Focalin; Focalin XR; M32RH9MFGP; Methyl D-phenidate; d-Methylphenidate; Attenade; Dex methylphenidate; d-threo-Methylphenidate; dex-methylphenidate; dexmethylphenidatum; dexmetilfenidato; threo-(+)-Methylphenidate; (+)-threo-Methylphenidate; 40431-64-9; CHEBI:51860; CHEMBL827; D-MPH; D-TMP; UNII-M32RH9MFGP; methyl (2R)-2-phenyl-2-[(2R)-piperidin-2-yl]acetate; methyl (2R)-phenyl[(2R)-piperidin-2-yl]acetate; methyl (R)-phenyl[(R)-piperidin-2-yl]acetate
|
| Indication |
Attention deficit hyperactivity disorder
[ICD11: 6A05]
|
Approved
|
[1]
|
| Structure |
|
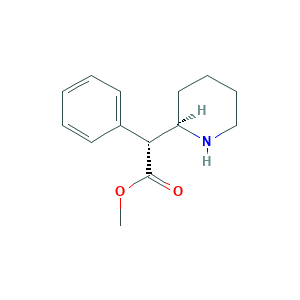 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
233.31 |
Topological Polar Surface Area |
38.3 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 154101
- ChEBI ID
-
- CAS Number
-
- Formula
- C14H19NO2
- Canonical SMILES
- COC(=O)C(C1CCCCN1)C2=CC=CC=C2
- InChI
- 1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3/t12-,13-/m1/s1
- InChIKey
- DUGOZIWVEXMGBE-CHWSQXEVSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


