| Synonyms |
Vistusertib; vistusertib (AZD2014); 0BSC3P4H5X; 1009298-59-2; 3-(2,4-bis((S)-3-methylmorpholino)pyrido[2,3-d]pyrimidin-7-yl)-N-methylbenzamide; 3-[2,4-Bis((3S)-3-methyLmorpholin-4-yl)pyrido-[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; 3-[2,4-Bis((3S)-3-methylmorpholin-4-yl)pyrido[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide; AZD 2014; AZD-2014; AZD2014; C25H30N6O3; CHEMBL2336325; UNII-0BSC3P4H5X; cc-551
|
| Cross-matching ID |
- PubChem CID
- 25262792
- PubChem SID
-
58097037
; 85072199
; 137085461
; 141539022
; 144115665
; 152159595
; 152258658
; 160647494
; 162011892
; 164045802
; 164149381
; 164158576
; 172650682
; 185990497
; 198984517
; 223366032
; 223389028
; 223702573
; 223705079
; 226633861
; 241383711
; 242060353
; 248835190
; 251962984
; 252214932
; 252450566
; 252543438
; 252552002
- CAS Number
-
- TTD Drug ID
- D0Z2UQ
- Formula
- C25H30N6O3
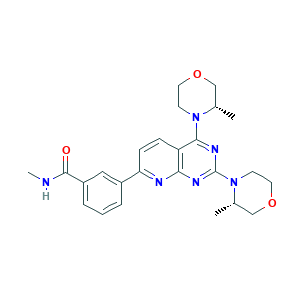
- Canonical SMILES
- CC1COCCN1C2=NC(=NC3=C2C=CC(=N3)C4=CC(=CC=C4)C(=O)NC)N5CCOCC5C
- InChI
- 1S/C25H30N6O3/c1-16-14-33-11-9-30(16)23-20-7-8-21(18-5-4-6-19(13-18)24(32)26-3)27-22(20)28-25(29-23)31-10-12-34-15-17(31)2/h4-8,13,16-17H,9-12,14-15H2,1-3H3,(H,26,32)/t16-,17-/m0/s1
- InChIKey
- JUSFANSTBFGBAF-IRXDYDNUSA-N
|