| General Information of Drug (ID:
DR1944) |
| Drug Name |
ICI-79,280
|
| Synonyms |
Hydroxytamoxifen; Trans-4-hydroxytamoxifen; Tamogel; Z-4-hydroxytamoxifen; trans-4-Hydroxytamoxifen; (E/Z)-4-Hydroxy Tamoxifen; (E/Z)-4-Hydroxytamoxifen; TAMOXIFEN, 4-HYDROXY-, (Z)-; (Z)-4-(1-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-phenylbut-1-en-1-yl)phenol; (Z)-4-Hydroxytamoxifen; 4-Hydroxytamoxifen; 4-Monohydroxytamoxifen; 4-OH-TAM; 65213-48-1; 95K54647BZ; BRN 4910749; C26H29NO2; CHEBI:44616; CHEMBL489; ICI 79,280; Ici 79280; MLS000069742; SMR000058939; UNII-95K54647BZ
|
| Indication |
Ductal carcinoma
[ICD11: 2E65]
|
Phase 2
|
[]
|
| Structure |
|
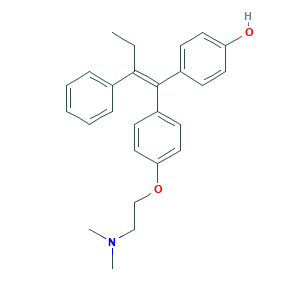 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
387.5 |
Topological Polar Surface Area |
32.7 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 449459
- PubChem SID
-
7494
; 835333
; 7889566
; 10300358
; 11532960
; 14719814
; 14719815
; 14805025
; 24895736
; 24895814
; 24899989
; 26757818
; 26759601
; 36891034
; 46393897
; 47208207
; 48243335
; 49688682
; 49846252
; 50005439
; 50065066
; 53788270
; 53789854
; 56422185
; 57404921
; 57570889
; 57654657
; 77095723
; 85788533
; 87219226
; 91613892
; 93165645
; 93167122
; 103169566
; 104640983
; 124799764
; 124893066
; 126639485
; 126671492
; 126685161
; 134341205
; 135021191
; 135065359
; 135610264
; 137156415
; 142089319
; 144208141
; 162226886
; 163414603
; 163687254
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09NST
- Formula
- C26H29NO2
- Canonical SMILES
- CCC(=C(C1=CC=C(C=C1)O)C2=CC=C(C=C2)OCCN(C)C)C3=CC=CC=C3
- InChI
- 1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25-
- InChIKey
- TXUZVZSFRXZGTL-QPLCGJKRSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


