Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2142) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Daptomycin
|
|||||
| Synonyms |
Dapcin; Daptomicina; Daptomycine; Daptomycinum; LY 146032; LY-146032; LY146032; (2S)-daptomycin; 103060-53-3; C72H101N17O26; Cidecin; Cubicin; DAPTOMYCIN; CHEBI:600103; N-decanoyl-L-tryptophyl-L-asparaginyl-N-[(3S,6S,9R,15S,18R,21S,24S,30S,31R)-3-[2-(2-aminophenyl)-2-oxoethyl]-24-(3-aminopropyl)-15,21-bis(carboxymethyl)-6-[(2R)-1-carboxypropan-2-yl]-9-(hydroxymethyl)-18,31-dimethyl-2,5,8,11,14,17,20,23,26,29-decaoxo-1-oxa-4,7,10,13,16,19,22,25,28-nonaazacyclohentriacontan-30-yl]-L-alpha-asparagine; SCHEMBL28102
|
|||||
| Indication | Methicillin-resistant staphylococcus infection [ICD11: 1D01] | Approved | [1] | |||
| Structure |
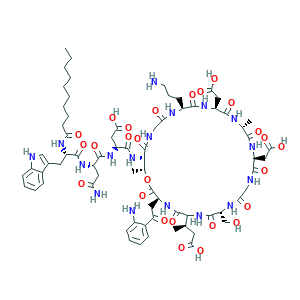 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 1620.7 | Topological Polar Surface Area | 702 | ||
| Heavy Atom Count | 115 | Rotatable Bond Count | 35 | |||
| Hydrogen Bond Donor Count | 22 | Hydrogen Bond Acceptor Count | 28 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

