| General Information of Drug (ID:
DR2178) |
| Drug Name |
Cephalothin
|
| Synonyms |
Cefalothin; Cefalotin (BAN); Cefalotina; Cefalotina [INN-Spanish]; Cefalotina fabra; Cefalotina fabra (TN); Cefalotine; Cefalotine [INN-French]; Cefalotinum; Cefalotinum [INN-Latin]; Cemastin; Cephalothin Monosodium Salt; Cephalothinum; Cephalotin; Coaxin; Keflin (TN); Averon-1; (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(acetyloxy)methyl]-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 3-(Acetoxymethyl)-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; 3-(Hydroxymethyl)-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid acetate; 3-ACETOXYMETHYL-8-OXO-7-(2-THIOPHEN-2-YL-ACETYLAMINO)-5-THIA-1-AZA-BICYCLO[4.2.0]OCT-2-ENE-2-CARBOXYLIC ACID; 3-Acetoxymethyl-7-(2-thienylacetamido)-3-cephem-4-carboxylic acid; 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 3-(hydroxymethyl)-8-oxo-7-(2-(2-thienyl)acetamido)-, acetate (ester); 6R-trans-3-((Acetyloxy)methyl)-8-oxo-7-((2-thienylacetyl)amino)-5-thia-1-azabicyclo(4.2.0)-oct-2-ene-2-carboxylic acid; 7-(2-(2-Thienyl)acetylamido)cephalosporanic acid; 7-(2-Thienylacetamido)cephalosporanic acid; 7-(Thiophene-2-acetamido)cephalosporanic acid; 7-(Thiophene-2-acetamido)cephalosporin; 7beta-(thiophen-2-ylacetamido)-3-acetoxymethyl-3,4-didehydrocepham-4-carboxylic acid
|
| Indication |
Infectious cystitis
[ICD11: GC00]
|
Approved
|
[1]
|
| Structure |
|
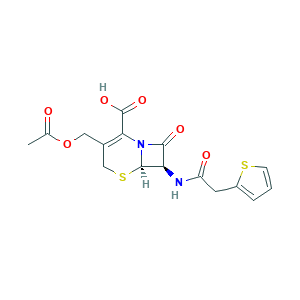 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
396.4 |
Topological Polar Surface Area |
167 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 6024
- PubChem SID
-
9963
; 602881
; 824703
; 7886646
; 7978894
; 8153752
; 11335272
; 11360511
; 11362964
; 11365526
; 11368088
; 11371283
; 11373877
; 11376250
; 11461483
; 11466747
; 11467867
; 11483898
; 11486594
; 11487937
; 11490148
; 11492084
; 11493924
; 14781066
; 16794138
; 29215241
; 29225038
; 46509079
; 47193756
; 47588840
; 47662113
; 47736307
; 47810601
; 47885259
; 48110301
; 48334326
; 48334327
; 48415743
; 50050997
; 50716459
; 51091939
; 57323124
; 81093187
; 84981763
; 85788476
; 87322650
; 103186843
; 104097230
; 104310899
; 124766006
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01PLN
- Formula
- C16H16N2O6S2
- Canonical SMILES
- CC(=O)OCC1=C(N2C(C(C2=O)NC(=O)CC3=CC=CS3)SC1)C(=O)O
- InChI
- 1S/C16H16N2O6S2/c1-8(19)24-6-9-7-26-15-12(14(21)18(15)13(9)16(22)23)17-11(20)5-10-3-2-4-25-10/h2-4,12,15H,5-7H2,1H3,(H,17,20)(H,22,23)/t12-,15-/m1/s1
- InChIKey
- XIURVHNZVLADCM-IUODEOHRSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


