| General Information of Drug (ID:
DR2185) |
| Drug Name |
Misonidazole
|
| Synonyms |
Misonidazol [INN-Spanish]; Misonidazole; Misonidazolum [INN-Latin]; Ro 7-0582; SR 1354; SRI 1354; 1-(2'-hydroxy-3'-methoxypropyl)-2-nitroimidazole; 1-(2-Hydroxy-3-methoxypropyl)-2-nitroimidazole; 1-(2-NITRO-1-IMIDAZOLYL)-3-METHOXY-2-PROPANOL; 1-methoxy-3-(2-nitro-1H-imidazol-1-yl)propan-2-ol; 1-methoxy-3-(2-nitroimidazol-1-yl)propan-2-ol; 13551-87-6; BRN 0613655; CCRIS 1160; EINECS 236-931-6; MLS003115361; NSC-261,037; NSC-261037; NSC261037; alpha-(Methoxymethyl)-2-nitro-1H-imidazole-1-ethanol
|
| Indication |
Discovery agent
|
Investigative
|
[1]
|
| Structure |
|
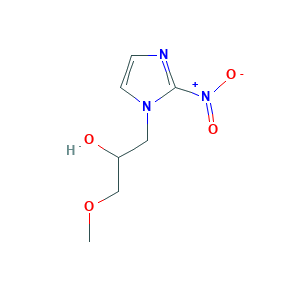 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
201.18 |
Topological Polar Surface Area |
93.1 |
| Heavy Atom Count |
14 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 26105
- CAS Number
-
- Formula
- C7H11N3O4
- Canonical SMILES
- COCC(CN1C=CN=C1[N+](=O)[O-])O
- InChI
- 1S/C7H11N3O4/c1-14-5-6(11)4-9-3-2-8-7(9)10(12)13/h2-3,6,11H,4-5H2,1H3
- InChIKey
- OBBCSXFCDPPXOL-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


