| Synonyms |
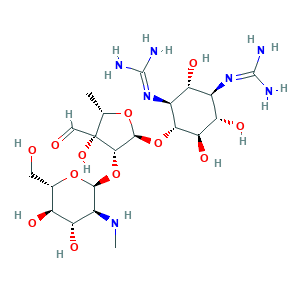
Streptomicina [Italian]; Streptomycin A; Streptomycin A sulfate; Streptomycin [INN:BAN]; Streptomycin sulfate; Streptomycin sulphate; Streptomycine; Streptomycinum; Streptomyzin [German]; Agrept; Agrimycin; Chemform; Estreptomicina [INN-Spanish]; Hokko-mycin; Neodiestreptopab; Strepcen; Y45QSO73OB; streptomycin; 2,4-Diguanidino-3,5,6-trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-alpha-L-glucopyranosyl)-3-C-formyl-beta-L-lyxopentanofuranoside; 57-92-1; CHEBI:17076; Caswell No. 804; Gerox; NSC-14083; UNII-Y45QSO73OB
|
| Cross-matching ID |
- PubChem CID
- 19649
- ChEBI ID
-
- CAS Number
-
- Formula
- C21H39N7O12
- Canonical SMILES
- CC1C(C(C(O1)OC2C(C(C(C(C2O)O)N=C(N)N)O)N=C(N)N)OC3C(C(C(C(O3)CO)O)O)NC)(C=O)O
- InChI
- 1S/C21H39N7O12/c1-5-21(36,4-30)16(40-17-9(26-2)13(34)10(31)6(3-29)38-17)18(37-5)39-15-8(28-20(24)25)11(32)7(27-19(22)23)12(33)14(15)35/h4-18,26,29,31-36H,3H2,1-2H3,(H4,22,23,27)(H4,24,25,28)/t5-,6-,7+,8-,9-,10-,11+,12-,13-,14+,15+,16-,17-,18-,21+/m0/s1
- InChIKey
- UCSJYZPVAKXKNQ-HZYVHMACSA-N
|