| DM Name |
DM ID |
PubChem ID |
Reaction |
DM Level |
REF |
|
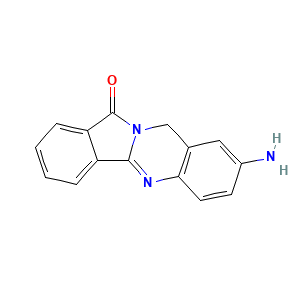
Daniquidone metabolite B1
|
DM017309
|
N. A. |
Oxidation
-
Hydroxylation |
1 |
[2]
|
|
Daniquidone metabolite B2
|
DM017310
|
N. A. |
Oxidation
-
Hydroxylation |
1 |
[2]
|
|
Daniquidone metabolite B3
|
DM017311
|
N. A. |
Oxidation
-
Hydroxylation |
1 |
[2]
|
|
Daniquidone metabolite B4
|
DM017312
|
N. A. |
Oxidation
-
Hydroxylation |
1 |
[2]
|
|
Daniquidone metabolite B5
|
DM017313
|
N. A. |
Oxidation
-
Hydroxylation |
1 |
[2]
|
|
Daniquidone metabolite B6
|
DM017314
|
N. A. |
Oxidation
-
Hydroxylation |
1 |
[2]
|
|
N-acetyl-batracylin
|
DM017308
|
N. A. |
Unclear |
1 |
[3]
|
|
|
|
|
|
|