| Synonyms |
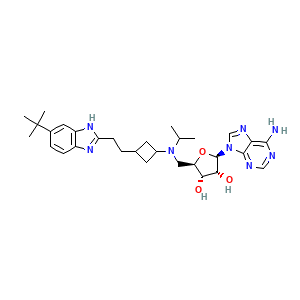
pinometostat; EPZ-5676; 1380288-87-8; EPZ5676; UNII-8V9YR09EF3; UNII-F66X4M38G5; 8V9YR09EF3; CHEMBL3087499; CHEMBL3414626; F66X4M38G5; 1380288-88-9; (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]imidazol-2-yl)ethyl)cyclobutyl)(isopropyl)amino)methyl)tetrahydrofuran-3,4-diol; Pinometostat, trans-; 5'-[{cis-3-[2-(5-Tert-Butyl-1h-Benzimidazol-2-Yl)ethyl]cyclobutyl}(Propan-2-Yl)amino]-5'-Deoxyadenosine
|
| Cross-matching ID |
- PubChem CID
- 57345410
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C6GK
- Formula
- C30H42N8O3
- Canonical SMILES
- CC(C)N(C[C@@H]1[C@H]([C@H]([C@@H](O1)N2C=NC3=C(N=CN=C32)N)O)O)C4CC(C4)CCC5=NC6=C(N5)C=C(C=C6)C(C)(C)C
- InChI
- InChI=1S/C30H42N8O3/c1-16(2)37(13-22-25(39)26(40)29(41-22)38-15-34-24-27(31)32-14-33-28(24)38)19-10-17(11-19)6-9-23-35-20-8-7-18(30(3,4)5)12-21(20)36-23/h7-8,12,14-17,19,22,25-26,29,39-40H,6,9-11,13H2,1-5H3,(H,35,36)(H2,31,32,33)/t17?,19?,22-,25-,26-,29-/m1/s1
- InChIKey
- LXFOLMYKSYSZQS-XKHGBIBOSA-N
|