| Synonyms |
Tozasertib; MK 0457; VX 680; VX6; L-001281814; MK-045; MK-0457; Tozasertib (USAN); VX-68; MK-0457, Tozasertib, VX680, VX-680; N-[4-[4-(4-methylpiperazin-1-yl)-6-[(5-methyl-1H-pyrazol-3-yl)amino]pyrimidin-2-yl]sulfanylphenyl]cyclopropanecarboxamide; N-(4-(4-(3-methyl-1H-pyrazol-5-ylamino)-6-(4-methylpiperazin-1-yl)pyrimidin-2-ylthio)phenyl)cyclopropanecarboxamide; Cyclopropanecarboxylic acid N-(4-((4-(4-methylpiperazin-1-yl)-6-(5-methyl-2H-pyrazol-3-ylamino)pyrimidin-2-yl)sulfanyl)phenyl)amide; Cyclopropane carboxylic acid{4-[4-(4-methyl-piperazin-1-yl)-6-(5-methyl-2H-pyrazol-3-ylamino)-pyrimidin-2-ylsulphanyl]-phenyl}-amide; Cyclopropanecarboxylic Acid {4-[4-(4-Methyl-Piperazin-1-Yl)-6-(5-Methyl-2h-Pyrazol-3-Ylamino)-Pyrimidin-2-Ylsulfanyl]-Phenyl}-Amide
|
| Cross-matching ID |
- PubChem CID
- 5494449
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02XNW
- Formula
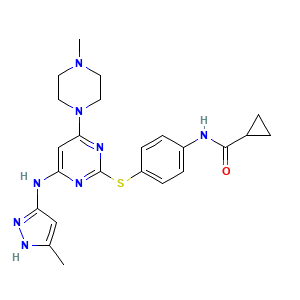
- C23H28N8OS
- Canonical SMILES
- CC1=CC(=NN1)NC2=CC(=NC(=N2)SC3=CC=C(C=C3)NC(=O)C4CC4)N5CCN(CC5)C
- InChI
- InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29)
- InChIKey
- GCIKSSRWRFVXBI-UHFFFAOYSA-N
|